5 Article 3
Multiproxy analysis exploring patterns of diet and disease in dental calculus and skeletal remains from a 19th century Dutch population
5.1 Introduction
Dental calculus has proven to be an excellent source of a wide variety of information about our past. The increased accessibility and advancement of methods in aDNA, paleoproteomics, and mass spectrometry, has expanded our ability to identify biomarkers of diet and disease on an increasingly large scale (Gismondi et al., 2020; Velsko et al., 2017; Warinner et al., 2014).
One such collection of biomarkers is alkaloids, a plant-derived group of compounds. Many alkaloids have important medicinal and psychoactive effects in humans, and their direct detection, or detection of their metabolites, is of great interest to archaeologists. Previous studies have successfully recovered alkaloids in archaeological contexts, including ceramics (Smith et al., 2018), pipes (Rafferty et al., 2012), human hair (Echeverría & Niemeyer, 2013; Ogalde et al., 2009), and even dental calculus employing both targeted (Eerkens et al., 2018) and untargeted approaches (Buckley et al., 2014; Gismondi et al., 2020). Especially nicotine, the principal alkaloid in tobacco leaves, has been widely studied in the archaeological record due to its apparent stability and ability to survive over long periods of time (Eerkens et al., 2018; Rafferty et al., 2012; Tushingham et al., 2013).
Alkaloids may enter the oral cavity via two pathways: (1) direct incorporation through ingestion of alkaloid-containing plants, whether deliberate or accidental; and (2) passive diffusion as alkaloids and other compounds are transferred from plasma to saliva, and then gradually secreted into the oral cavity through the salivary glands in the hours-to-days following ingestion (Cone & Huestis, 2007). The second pathway allows the identification of parent compounds that do not enter the mouth (e.g. injection), as long as they, or their metabolites, are excreted through the saliva, thus eventually entering the oral cavity.
Many of the components involved in the formation and growth of dental calculus originate from oral fluid. Proteins, bacteria, salts and other compounds are transferred from saliva to biofilms on the tooth surface (Jin & Yip, 2002; White, 1997). This may also allow various alkaloids of dietary and medicinal origin to become incorporated in dental plaque. Dental plaque undergoes frequent mineralisation events, ultimately causing the entrapped alkaloids and their metabolites to become preserved within the dental calculus. Barring intentional or accidental removal of the calculus during life, burial, excavation, and post-excavation cleaning, the alkaloids can then be detected by various methods to show a record of consumption during life. Because drugs may be transferred from plasma to saliva, there is often a close correlation between drugs detected in oral fluid and blood, though there are differences in detected concentrations (Cone & Huestis, 2007; Milman et al., 2011; Wille et al., 2009). This was also shown to be true for dental calculus and blood (Sørensen et al., 2021), making dental calculus a potentially useful substance for detecting ancient alkaloids and other dietary compounds.
In this study we use a ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method that was developed in a previous study on dental calculus from cadavers and validated by comparing the results to compounds detected in the blood of the same individuals (Sørensen et al., 2021). All compounds that were detected in the blood were also detected in dental calculus, with additional compounds present in dental calculus that were not present in blood, suggesting that dental calculus represents a comprehensive history of consumption over a long period of time (Sørensen et al., 2021). We were able to detect both parent compounds and metabolites, including caffeine, nicotine, theophylline, and cotinine, in the dental calculus of individuals from a 19th century Dutch population from Middenbeemster. By detecting these compounds we are able to show the consumption of tea and coffee and smoking of tobacco on an individual scale, which is also confirmed by historic documentation and identification of pipe notches in the dentition.
5.2 Materials
The sample consists of 41 individuals from Middenbeemster, a 19th century rural Dutch site. The village of Middenbeemster and the surrounding Beemsterpolder was established in the beginning of the 17th century, when the Beemster lake was drained to create more farmland, mainly for the cultivation of cole seeds (de Vries 1978). In 1615, a decision was made to build a church, and construction started in 1618 (Hakvoort 2013). The excavated cemetery is associated with the Keyserkerk church, where the inhabitants of the Middenbeemster village and the surrounding Beemsterpolder were buried between AD 1615 and 1866 (Lemmers et al., 2013). Archival documents are available for those buried between AD 1829 and 1866, when the majority of individuals were interred. The main occupation of the inhabitants was dairy farming, consisting largely of manual labour prior to the industrial revolution (Aten et al., 2012; Palmer et al., 2016).
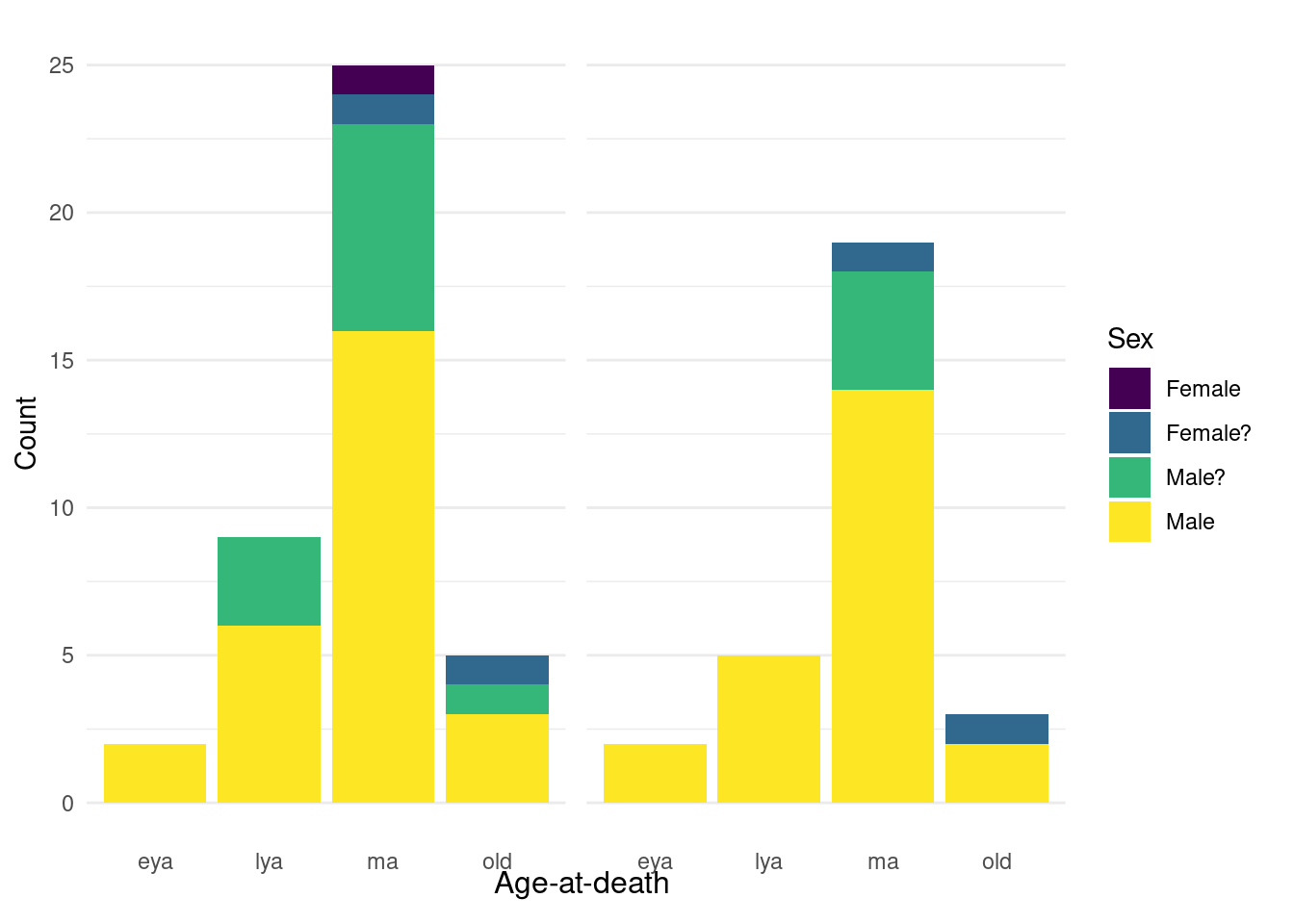
For our sample, we preferentially selected males from the middle adult age category (35-49 years) to minimise the effect of confounding cultural and biological factors. Previous research on Middenbeemster has shown a gendered division of labour (Palmer et al., 2016), and there are biological differences in dental calculus formation and drug metabolism that are related to age and sex (Huang et al., 2023; Uno et al., 2017; White, 1997). The sample consists of 27 males, 11 probable males, 2 probable females, and 1 female (Figure 5.1). We selected males due to a higher occurrence of pipe notches and dental calculus deposits than females (unpublished observation).

5.3 Methods
5.3.1 Skeletal analysis
Demographic and pathological analyses were conducted in the Laboratory for Human Osteoarchaeology at Leiden University. Sex was estimated using cranial and pelvic morphological traits (Buikstra & Ubelaker, 1994). Age-at-death was estimated using dental wear, auricular and pubic surface appearance, cranial suture closure, and epiphyseal fusion (Brooks & Suchey, 1990; Buckberry & Chamberlain, 2002; Buikstra & Ubelaker, 1994; Lovejoy et al., 1985; Meindl & Lovejoy, 1985), and divided into the following categories: early young adult (18-24 years), late young adult (25-34 years), middle adult (35-49 years), old adult (50+ years). Preservation was visually scored on a four-stage scale (excellent, good, fair, poor) based on the surface condition of the bones and the extent of taphonomic degradation.
5.3.1.1 Paleopathology
Pathological conditions and lesions that occur frequently in the population were included in the analysis. Data were dichotomised to presence/absence to allow for statistical analysis. Osteoarthritis was considered present in cases where eburnation was visible on one or more joint surfaces. Vertebral osteophytosis is identified by marginal lipping and/or osteophyte formation on the margin of the superior and inferior surfaces of the vertebral body. Cribra orbitalia was diagnosed based on the presence of pitting on the superior surface of the orbit. No distinction was made between active or healing lesions. Degenerative disc disease, or spondylosis, is identified as a large diffuse depression of the superior and/or inferior surfaces of the vertebral body (Rogers, 2000). Schmorl’s nodes are identified as any cortical depressions on the surface of the vertebral body. Data on chronic maxillary sinusitis from Casna et al. (2021) were included in this study to assess the relationship between upper respiratory diseases with environmental factors (i.e. tobacco smoke, caffeine consumption). Lesions associated with chronic maxillary sinusitis as defined by Boocock et al. (1995) were recorded for each individual and classified as “pitting”, “spicule-type bone formation”, “remodeled spicules”, or “white pitted bone”. chronic maxillary sinusitis was scored as absent when the sinus presented smooth surfaces with little or no associated pitting.
5.3.1.2 Dental pathology
Caries ratios were calculated by dividing the number of lesions by the number of teeth scored, resulting in a single caries ratio per individual. If the surface where the lesion originated is not visible, i.e. if the lesion covered multiple surfaces, this was scored as “crown”. Calculus indices were calculated according to Greene and colleagues (2005). Calculus was scored with a four-stage scoring system (0-3) to score absent, slight, moderate, and heavy calculus deposits (Brothwell, 1981) on the lingual, buccal (and labial), and interproximal surfaces of each tooth. Only one score was used for the combined interproximal surfaces, resulting in three scores per tooth (when surfaces are intact), and four calculus indices per individual; upper anterior, upper posterior, lower anterior, lower posterior. Each index was calculated by dividing the sum of calculus scores for each surface by the total number of surfaces scored in each quadrant. If a tooth could not be scored on all three surfaces, the tooth was not included (Greene et al., 2005). Periodontitis was scored on a visual four-stage (0-3) scoring system according to distance from cemento-enamel junction of each tooth to alveolar bone (Maat & Mastwijk, 2005).
5.3.2 Calculus sampling
Where possible, we used material that had already been sampled for a previous study to prevent unnecessary repeated sampling of individuals. Calculus from the previous study was sampled in a dedicated ancient DNA laboratory at the Laboratories of Molecular Anthropology and Microbiome Research in Norman, Oklahoma, U.S.A, using established ancient DNA protocols. More details on the methods can be found in the published articles (Ziesemer et al., 2015, 2018). Of the 41 individuals that were originally included in our sample, 29 were replicated in a separate analysis only using calculus from the previous study.
New dental calculus samples were taken under sterile conditions in a positive pressure laminar flow hood in a dedicated dental calculus lab at Leiden University. The surface of the tooth was lightly brushed with a sterile, disposable toothbrush to get rid of surface contaminants. A sterile dental curette was then used to scrape calculus from the tooth onto weighing paper, which was transferred to 1.5 ml Eppendorf tubes. All calculus samples were sent to the Department of Forensic Medicine at Aarhus University for ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) analysis.
5.3.3 UHPLC-MS/MS
The list of targeted compounds included both naturally occurring compounds known to have been used in the past, as well as synthetic modern drugs that did not exist at the time (e.g. Fentanyl, MDMA, Amphetamine). These were part of the toxicology screening for the original method (Sørensen et al., 2021), developed on cadavers. In our study they serve as an authentication step, as their presence in archaeological samples could only be the result of contamination.
Briefly, samples of dental calculus were washed three times each with one mL of methanol (MeOH), to remove surface contaminants. The wash solutions were collected separately. The solvent was evaporated and the residues were dissolved in 50 µL 30% MeOH. The washed calculus was homogenized in presence of 0.5 M citric acid using a lysing tube with stainless steel beads. Following one hour of incubation the dissolution extract was cleaned by weak and strong cation-exchange. After evaporation of the elution solvent the residue was dissolved in 50 µL 30% MeOH. The final extracts obtained from washing and dissolution of the dental calculus were analysed by UHPLC-MS/MS using a reversed-phase biphenyl column for chromatography. To obtain quantitative results, isotope dilution was applied. For more details about the method and validation, see the original study by Sørensen and colleagues (2021).
5.3.4 Statistical analysis
All compounds and pathological conditions/lesions were converted to a presence/absence score. Pearson product-moment correlation was applied to the dichotomised pathological lesions (point-biserial correlation), compound concentrations, calculus indices, and caries ratios to explore relationships paired continuous-continuous variables and paired continuous-binary variables. Compound concentrations were then dichotomised to presence/absence, and the caries ratio and calculus index for each individual were converted to an ordinal score from 0 to 4 by using quartiles. Polychoric correlation was applied to the paired dichotomous variables and dichotomous-ordinal variables.
All statistical analysis was conducted in R version 4.3.2 (2023-10-31), Eye Holes, (R Core Team, 2020). Data wrangling was conducted with the tidyverse (Wickham et al., 2019) and visualisations were created using ggplot2 (Wickham, 2016). Polychoric correlations were calculated with the psych package (Revelle, 2022).
5.4 Results
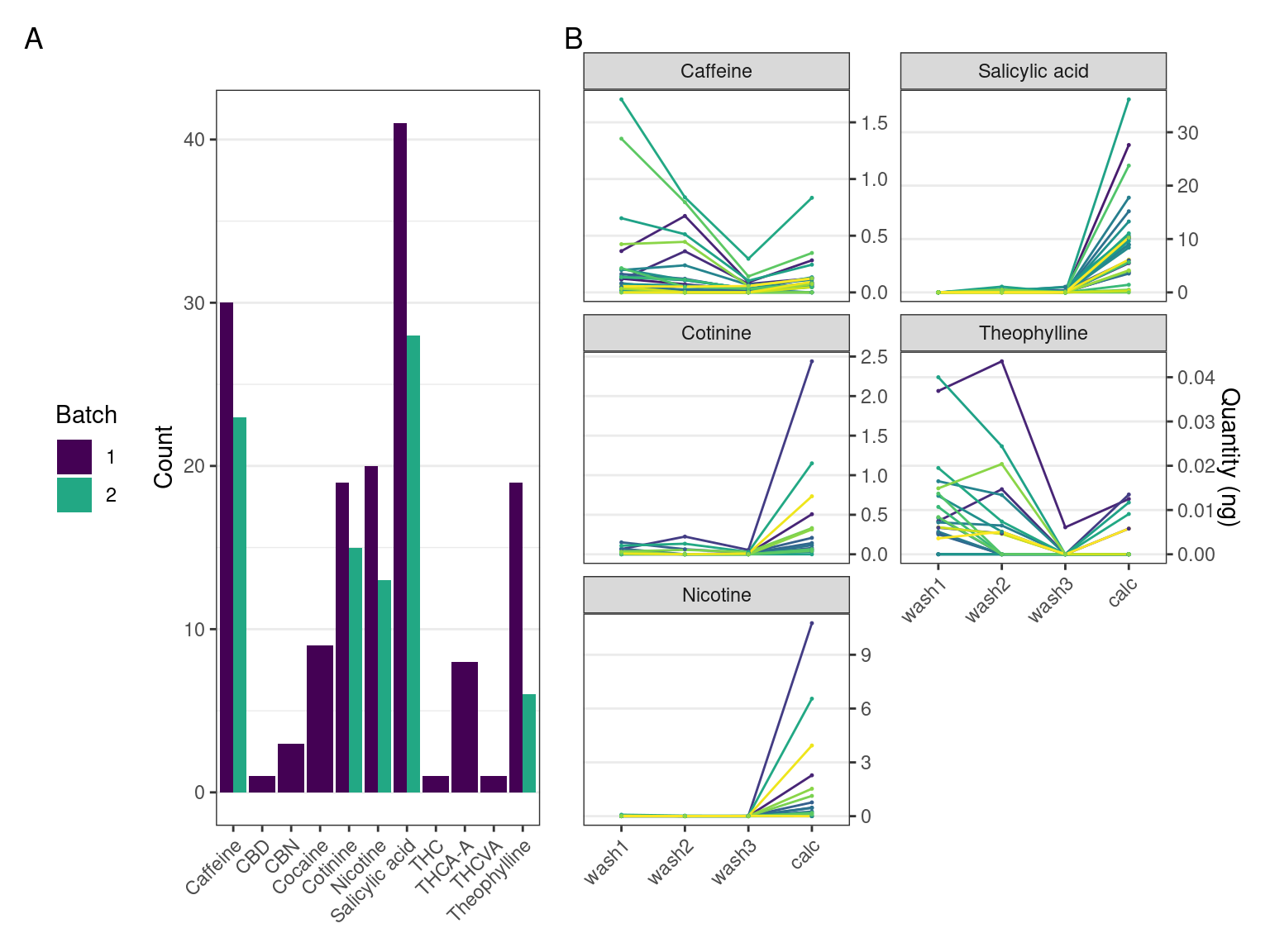
Multiple compounds were detected in the dental calculus samples. Compounds detected at a lower concentration than the lower limit of quantitation (LLOQ) were considered not present. Not all the compounds detected in the first batch could be replicated in the second batch (Table 5.1). For a full list of targeted compounds, see Supplementary Material.
| Compound | Batch 1 | Batch 2 | LLOQ |
|---|---|---|---|
| CBD | TRUE | FALSE | 0.050 |
| CBN | TRUE | FALSE | 0.050 |
| Caffeine | TRUE | TRUE | 0.050 |
| Cocaine | TRUE | FALSE | 0.025 |
| Cotinine | TRUE | TRUE | 0.050 |
| Nicotine | TRUE | TRUE | 0.100 |
| Salicylic acid | TRUE | TRUE | 0.500 |
| THC | TRUE | FALSE | 0.100 |
| THCA-A | TRUE | FALSE | 0.025 |
| THCVA | TRUE | FALSE | 0.010 |
| Theophylline | TRUE | TRUE | 0.010 |
The pattern we expect to see in authentic compounds representing compounds trapped within the dental calculus, is a reduction in the quantity from wash 1 to wash 3 as potential surface contaminants are washed off, and then a spike in the final extraction when entrapped compounds are released and detected.
Most plots show a large increase in extracted mass of a compound between the calculus wash extracts (wash 1-3) and the dissolved calculus (calc). Most samples containing theophylline and caffeine had the largest quantity of the compound extracted from the first wash, then decreasing in washes 2 and 3. There is an increase between wash 3 and the dissolved calculus in all samples. The patterns are consistent across batches 1 and 2. Nicotine and cotinine have the same relative quantities in the samples, i.e., the sample with the highest extracted quantity of nicotine also had the highest extracted quantity of cotinine (Figure 5.2).
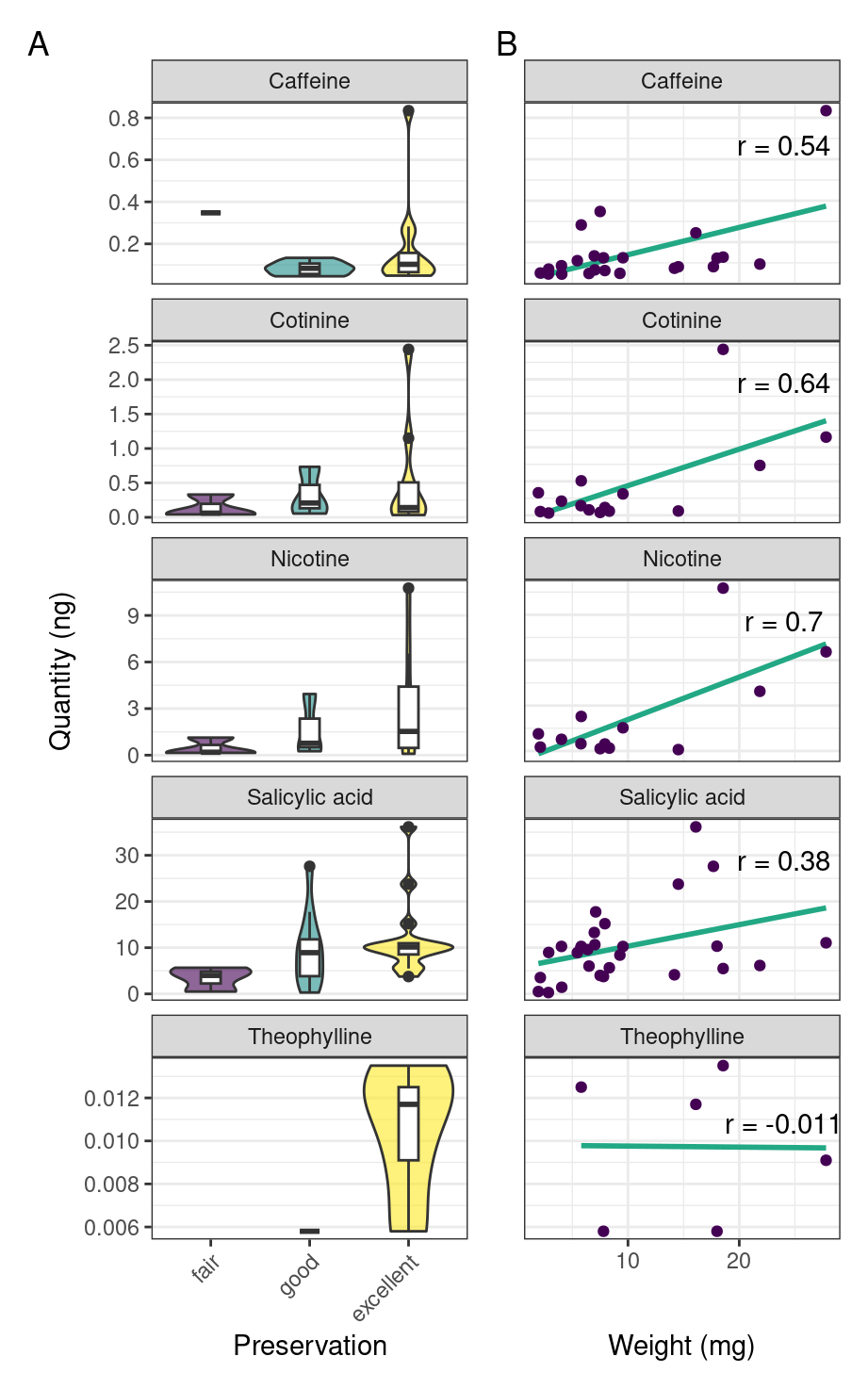
To see if preservation of the skeletal remains had any effect on the detection of compounds, we compare extracted quantities of compounds to the various levels of skeletal preservation. Our results from batch 2 suggest that detection of a compound may be linked to the preservation of the skeleton, with better preservation leading to increased extraction quantity (Figure 5.3A). We also find a weak positive correlation between the weight of the calculus sample and the quantity of compound extracted from the calculus (Figure 5.3B).
The presence of pipe notch(es) in an individual and concurrent detection of nicotine and/or cotinine is used as a crude indicator of the accuracy of the method. Only males were used in accuracy calculations, as pipe notches are ubiquitous in males, but not in females. In batch 2, the method was able to detect some form of tobacco in 14 of 25 individuals with a pipe notch (56.0%). When also considering correct the absence of a tobacco alkaloid together with the absence of a pipe notch, the accuracy of the method is 59.3%. Accuracy in the old adult age category is 100.0%, but with only 2 individuals.
One individual—an old adult, probable female—was positive for both nicotine and cotinine, and had no signs of a pipe notch.
5.4.1 Correlations between detected alkaloids and diseases
For further statistical analyses, only the UHPLC-MS/MS results from batch 2 were used, as batch 1 had multiple compounds that were not detected in batch 2 and may have been contaminated.
| Caries | Nicotine | SA | Calculus | PN | Theophylline | Caffeine | Cotinine | |
|---|---|---|---|---|---|---|---|---|
| OA | -0.12 | -0.07 | 0.21 | 0.07 | 0.14 | 0.28 | 0 | -0.07 |
| VOP | -0.09 | -0.16 | 0.34 | 0.06 | 0.25 | -0.06 | 0.01 | -0.13 |
| SN | -0.24 | 0.16 | 0.09 | 0.09 | 0.17 | 0.24 | 0.16 | 0.09 |
| DDD | 0 | 0 | 0.19 | -0.39 | -0.08 | 0.31 | 0.06 | -0.01 |
| CO | 0.06 | -0.05 | 0.2 | 0.14 | -0.2 | -0.11 | 0.19 | -0.06 |
| CMS | -0.19 | 0.28 | 0 | -0.27 | 0.03 | 0.19 | 0.36 | 0.22 |
| Caries | -0.2 | -0.36 | -0.15 | -0.17 | -0.21 | 0 | -0.22 | |
| Nicotine | -0.21 | 0.01 | -0.01 | 0.43 | 0.14 | 0.98 | ||
| SA | 0.14 | 0.37 | 0.04 | 0.17 | -0.17 | |||
| Calculus | 0.13 | -0.15 | -0.13 | 0.03 | ||||
| PN | -0.16 | 0.18 | -0.01 | |||||
| Theophylline | 0.51 | 0.36 | ||||||
| Caffeine | 0.08 |
Point-biserial correlation was conducted on paired continuous and dichotomous variables, to see if any relationships exist between extracted concentrations and other variables. The strongest point-biserial (Pearson) correlation correlations were a near-perfect positive correlation between cotinine and nicotine (0.98), and moderate correlations between theophylline and nicotine (0.43), caffeine and theophylline (0.51) (Table 5.2).
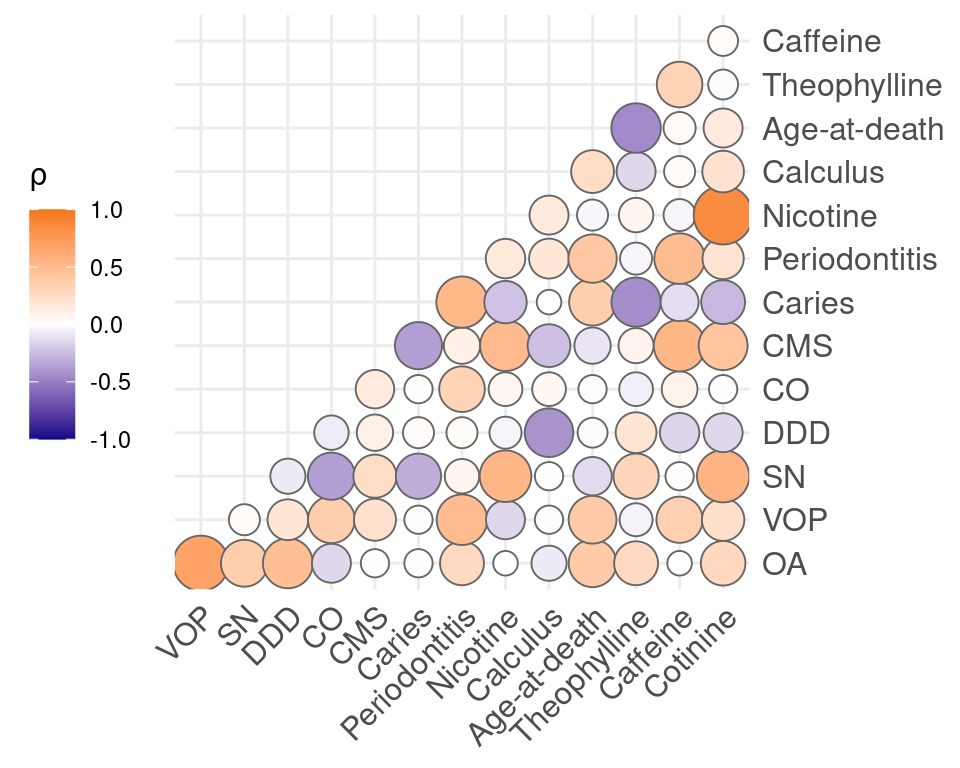
Polychoric correlation was conducted on the dichotomised compounds and pathological conditions, as well as the discretised dental diseases. Salicylic acid was removed due to its ubiquitous presence in the sample, and is likely to cause spurious correlations. Strong correlations were found between cotinine and nicotine (0.85). Moderate correlations were found between OA and DDD (0.47), VOP and periodontitis (0.49), SN and cotinine (0.56), DDD and calculus (-0.42), CMS and caffeine (0.53), caries and periodontitis (0.52), periodontitis and VOP (0.49), periodontitis and age-at-death (0.41), nicotine and SN (0.53), calculus and DDD (-0.42), age-at-death and theophylline (-0.45), theophylline and age-at-death (-0.45), caffeine and periodontitis (0.49), cotinine and CMS (0.43). Remaining correlations were weak or absent (Figure 5.4). Correlations with age will be depressed because age was largely controlled for in the sample selection.
5.5 Discussion
In this study we were able to extract and identify multiple alkaloids and salicylic acid from the dental calculus of individuals from Middenbeemster, a 19th century Dutch archaeological site. We applied ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) using a method that was validated by co-occurrence of drugs and metabolites in dental calculus and blood (Sørensen et al., 2021). Here we have shown that the method can also be successfully applied to archaeological dental calculus. We extend findings from previous studies on alkaloids in archaeological samples by detecting multiple different alkaloids in dental calculus, including nicotine, cotinine, caffeine, theophylline, and salicylic acid. The detection of these compounds was solidified in a replication analysis on different samples from the same individuals. Cocaine and multiple cannabinoids were also detected during the first analysis, but were not replicated. We contextualize these findings within the historical and archaeological evidence for consumption of these drugs and dietary compounds.
Nicotine and its principal/main metabolite, cotinine, were strongly positively correlated, both in concentration and presence/absence in individuals (Table 5.2 and Figure 5.4). The detection of nicotine and cotinine is not surprising, as pipe-smoking in the Beemsterpolder is well-documented in the literature (Aten et al., 2012; Bouman, 2017), and visible on the skeletal remains as pipe notches (Lemmers et al., 2013). There is also documented medicinal use of nicotine in the Beemsterpolder, where a tobacco-smoke enema was used for headaches, respiratory problems, colds, and drowsiness from around 1780 to 1830 (Aten et al., 2012). In our sample, we also detected nicotine and cotinine (replicated) in an old adult, probable female individual. In this particular case it is unlikely that the compounds entered the dental calculus through pipe-smoking, as the individual had no visible pipe notches; more likely the tobacco entered through an alternate mode of consumption, secondhand smoke, or the aforementioned tobacco-smoke enema.
Theophylline and caffeine were positively correlated in our samples, though to a lesser extent than nicotine and cotinine, so we are unable to determine if they originated from the same source (Table 5.2 and Figure 5.4). Caffeine and theophylline have very similar chemical structures, so we expect they would experience similar rates of incorporation and degradation, allowing us to interpret the ratio and correlations between the compounds. Caffeine is present in coffee, tea, and cocoa beans, with concentrations slightly higher in coffee (Bispo et al., 2002; Chin et al., 2008; Srdjenovic et al., 2008; Stavric et al., 1988). Theophylline is present in both coffee beans and tea leaves, but in negligible quantities (Stavric et al., 1988). It is also a primary metabolite of caffeine produced by the liver. Given the low correlation, there are likely multiple sources of caffeine and theophylline in the population, with tea and coffee being the most obvious.
Tea consumption had become widespread in the Netherlands by 1820, reaching all parts of society (Nierstrasz, 2015, p. 91). Historically, we also know that both tea and coffee were consumed in the Beemsterpolder during the 19th century. ‘Theegasten’ (teatime) was a special occasion occurring from 15.00-20.00 hours, where tea was served along with the evening bread (Schuijtemaker, 2011). Many households also owned at least one coffee pot and tea pot (Bouman, 2017). Distinguishing between tea, coffee, and chocolate may be possible by also including theobromine and comparing ratios of the compounds, as theobromine is present in higher quantities in chocolate compared to caffeine and theophylline (Alañón et al., 2016; Bispo et al., 2002; Stavric et al., 1988). However, In addition to oral factors affecting alkaloid uptake in dental calculus, there is some indication that theobromine does not preserve well in the archaeological record (Velsko et al., 2017), and frequent consumption of all three items would be difficult to parse. Additionally, we do not understand well enough the effect of the burial on these specific compounds, and the original concentration of the compounds in plants can be quite variable (King et al., 2017).
Salicylic acid was found in all but one individual in our sample. It can be extracted from the bark of willow trees, Salix alba, and has long been used for its pain-relieving properties (Bruinsma, 1872, p. 119). It is also present in many plant-based foods (Duthie & Wood, 2011; Malakar et al., 2017), including potatoes, which were a staple of the Beemsterpolder diet (Aten et al., 2012). The extracted quantity from our samples decreased over the three washes, followed by a sharp increase in the final calculus extraction, which is what we would expect to see if the salicylic acid was incorporated during life (Figure 5.2). It is important to note that, especially with salicylic acid, there is a possibility for the compound to enter the calculus through contact with the surrounding soil. Salicylic acid is a very mobile organic acid (Badri & Vivanco, 2009; Chen et al., 2001) and the ubiqutous presence in our samples may be explained by the compound leching into the dental calculus from the burial environment. We can therefore not confidently rule out environmental contamination without analysing samples from the surrounding soil.
Cannabinoids—specifically THC, THCA-A, THCVA, CBD, CBN—were found in the first batch, but none were replicated in the second batch. Medicinal use of cannabinoids has been well-established in Europe since Medieval-times, and it was also grown in the Netherlands (Bruinsma, 1872). Administration was most common in the form of concoctions containing various portions of the cannabis plant for ingestion; not until the late 19th century did it become recommended to smoke it for more immediate effects (Clarke, 2013). Dutch medicinal preparations were used to treat a variety of ailments and symptoms, including pain, inflammation, various stomach ailments, gout, and joint pains (Clarke, 2013). Because cannabinoids have an affinity for protein-binding, they are less likely to diffuse from serum to saliva (Cone & Huestis, 2007). This may make them difficult to detect in dental calculus unless the cannibinoids were consumed orally; even then, the overall instability of some cannabinoids could also limit detection (Lindholst, 2010; Sørensen & Hasselstrøm, 2018). Given the lack of replication, we cannot with security confirm that cannabis was used by the Beemster population.
Despite many of our sampled individuals having lived during the height of the opium era in the Netherlands (Macht, 1915), none of the targeted opioids (morphine, codeine, thebaine, papaverine, norcodeine, noscapine) were detected. The absence of opioids could be a result of the people ascribing more to the “traditional” rather than “scientific” medicine, although laudanum and another opium containing concoction was part of the “traditional” medicine in the Netherlands (Leuw & Marshall, 1994), including Middenbeemster (Aten et al., 2012). It was also generally considered a drug of the upper class (Scheltema, 1907), and may have been more common in urban centers. The absence could also be attributed to postmortem degradation. It has been shown that, while morphine is abundant in opium, it degrades rapidly. Thebaine and papaverine are more resistant to various ageing processes (Chovanec et al., 2012), however, these were also absent from our samples.
The only strictly modern compound (at least in a European context) detected in the sample was cocaine, which was detected in the first batch of samples. Our sample is derived from an early–mid 19th century population, and cocaine was isolated in 1860 by Albert Niemann, and entered popular medical practice in 1884. Coca arrived in Europe as early as 1771, but as botanical specimens rather than for consumption, and there were also issues importing enough viable specimens of coca for cocaine extraction (Abduca, 2019, p. 108; Mortimer, 1901, p. 179). This would have been the first case of coca-leaf-consumption in Europe; however, we were unable to replicate any of the cocaine results in the second batch. We suspect that the original detection of cocaine was a result of lab contamination during analysis.
We explored the relationship between detected compounds and various skeletal indicators, such as pathological and dental lesions, preservation, and pipe notches. We found some evidence to suggest that preservation of the skeleton influences the recovery of compounds from the dental calculus, with well-preserved skeletons potentially serving as a better target for sampling.
We found a positive correlation between CMS and nicotine, which may be indicative of the impact tobacco smoking had on the respiratory health of the Beemster inhabitants. Tobacco smoke may play a significant role in diseases of the upper respiratory tract, including chronic maxillary sinusitis (Reh et al., 2012). Although the mechanisms by which smoking increases the risk of infections is not fully understood, solid evidence has been presented linking tobacco smoke to increased mucosal permeability and impairment of mucociliary clearance (Arcavi & Benowitz, 2004). Such changes, together with an altered immunologic response, are thought to predispose to the development of chronic maxillary sinusitis (Slavin et al., 2005).
We also observed a moderate positive correlation between chronic maxillary sinusitis and caffeine which contradicts previous research linking chronic coffee consumption with a positive effect on the respiratory system, suggesting a preventive association between caffeine intake and pneumonia (e.g. Alfaro et al., 2018; Kondo et al., 2021). However, while the lower respiratory tract seems to benefit from chronic coffee consumption, it is possible that elevated caffeine intake impacts mucosal moisture due to its dehydrating effect (Maughan & Griffin, 2003), thereby exposing individuals to greater risk of sinus infection.
The detection of nicotine in dental calculus has previously been presented by Eerkens and colleagues (2018) in two individuals from pre-contact California. They also targeted caffeine, cotinine, and theophylline in their samples, but were unable to detect any of them. It remains to be seen whether this is due to differences in methods used, or due to our samples being more recent. They also suggest that the choice of tooth for sampling may impact the detection of certain compounds, as the incorporation in dental calculus may depend on the mode of consumption. Tobacco smokers may have more nicotine present in calculus on incisors, whereas tobacco chewers may have more on molars (Eerkens et al., 2018). However, sampling may not be limited to mode of consumption. The presence of cotinine suggests that the excretion of a compound after being metabolised in the body is also a source of deposition, and that deposition of alkaloids in dental calculus can occur both on the way into the body, i.e. during consumption, and on the way out, i.e. disposal of waste products via saliva secretion into the mouth. Especially mucin-rich saliva from the sublingual and submandibular glands preferentially binds toxins (Dodds et al., 2005), and since these glands are located closest to the lower incisors, they may be the most effective target for these studies. This has yet to be systematically tested in archaeological dental calculus. Because we homogenised samples from multiple teeth of an individual, we were unable to test the effect of oral biogeography. It is also possible that resident microflora within biofilms contribute to alkaloid breakdown and that the presence of caffeine and nicotine metabolites following direct ingestion can be explained by this pathway. However, the literature on biofilm biodegradation of alkaloids is limited, and in vitro studies have only found minimal contributions by certain oral bacteria in isolation (Cogo et al., 2008; Sun et al., 2016); it is possible that a larger role is played by oral bacteria within larger, more metabolically active communities, e.g. biofilms (Takahashi, 2015).
Targeting individuals with moderate-to-large calculus deposits likely biased our sample, as the presence of calculus may increase the risk of premature death (Yaussy & DeWitte, 2019). Additionally, periodontal disease (often linked to the presence of calculus) is a risk-factor for respiratory diseases, if periodontal and respiratory pathogens enter the bloodstream (Azarpazhooh & Leake, 2006; Scannapieco, 1999; Scannapieco & Ho, 2001). In our sample, the percentage of chronic maxillary sinusitis (37.0%) is lower than in another (more representative) male sample (44.1%) (Casna et al., 2021), and the caries percentage is similarly lower in our sample (17.6%) than a more representative sample (22.9%) (Lemmers et al., 2013).
We used the presence/absence of a pipe notch and concurrent detection of tobacco as a crude estimate of the accuracy of the method, which we found to be around 59.3%. This is a very rough estimate, as the presence of a pipe notch is likely not a perfect indicator of whether or not someone consumed tobacco. Dental calculus is also more transient than for example bone, as it can become dislodged during life, intentionally or unintentionally, eliminating all trace of the alkaloids consumed prior to its removal.
Following burial, compound stability over time will play a large role, as will microbial degradation of compounds by bacteria and fungi in soil (Liu et al., 2015), as well as the soil environment, such as temperature, pH, and oxygen availability (Lindholst, 2010; Mackie et al., 2017).
Due to this, quantitation of the detected compounds may have limited value in archaeological samples due to degradation, and will greatly affect our correlations related to concentration. The detected quantity of a compound will also depend on the quantity in dental calculus during life, which is largely controlled by the quantity consumed, how often the calculus was disrupted/removed, metabolic breakdown of the compound, and inter- and intra-individual factors related to stages of biofilm maturation (Lustmann et al., 1976; Velsko et al., 2019; Zijnge et al., 2010). In short, this means it is not really possible to equate the absence of a compound as evidence for the absence of consumption, which complicates the interpretation of our results. We have attempted to minimise errors occurring due to this limitation by including a relatively large sample of individuals and replicating our analysis. Although, given the relatively low detection rate seen in tobacco, this remains a major limitation, and will likely be compounded by increasing antiquity of the samples.
Future studies should explore how sampling from various types of teeth and their position in the mouth affects the probability of a compound becoming entrapped in dental calculus. This may also be related to properties within the oral cavity, as well as chemical properties of the compounds, which facilitate or reduce the incorporation-potential, and which incorporation pathways are more likely for a given compound.
We only targeted drugs that were included in the forensic toxicological screenings, and therefore only covered a limited number of the potential compounds that could be of interest for exploring past diets and medicinal treatments. The list of targeted compounds can be expanded as we discover more potential targets based on which specific compounds/metabolites are more likely to be incorporated and preserved in dental calculus.
There is an increasing interest in using oral fluid as a means of detecting alkaloids in living individuals due to the non-invasive nature of the testing compared to blood and urine sampling (Cone, 1993; Valen et al., 2017). These in vivo studies are a valuable source of method validation and can help determine the feasibility of detecting certain alkaloids in oral fluid and, subsequently, dental calculus. Archaeologists, though, will likely be responsible for exploring dental-calculus-specific incorporation and retention of alkaloids, as well as their long-term preservation in the burial environment. Finally, following our experience with salicylic acid, we encourage all future studies to ensure that a control sample is taken from the soil, either from the soil surrounding the individual, or, ideally, directly from the skeletal remains. This should preferably happen before cleaning, but there will often be soil left over in cavities (e.g. nasal cavity, orbit, auditory meatus).
While a major limitation is the uncertainty surrounding whether or not a compound is actually absent, the power of the method lies in the ability to detect dietary and other compounds that were incorporated via multiple consumption pathways that are not detected by other methods. Taking tobacco consumption as an example; while pipe notches are a useful way to identify tobacco consumption, pipe smoking was not the only mode of tobacco consumption, with others including chewing, drinking, cigars, and snuff (Goodman, 1994, p. 67). Pipe-smoking was mainly practised by males (Eerkens et al., 2018; Lemmers et al., 2013), so methods like the one presented here are suitable for exploring tobacco consumption in an entire society, rather than a trivial subset of past populations. Combined with other methods, it can also give us a more complete picture of dietary patterns and medicinal/recreational plant-use in the past by capturing multiple possible incorporation pathways of dietary (and other) compounds.
5.6 Conclusion
This preliminary study outlines the benefits of using calculus to target a variety of compounds that could have been consumed as medicine or diet. This method allows us to directly address specific individuals, which can be especially useful in individuals that are not always well-documented in historic documentation, such as rural communities, children and women. We also show that there are many limitations that will need to be addressed going forward with this type of analysis, and stress the need for more systematic research on the consumption of alkaloid-containing items and their subsequent concentration and preservation in dental calculus, in addition to how mode of consumption may affect concentrations on different parts of the dentition. Another limitation of dental calculus as a medium is the inter- and intra-individual variability of its formation and the many factors that can influence incorporation and retention of molecules and particles; however, in the absence of hair and serum (quite uncommon in archaeology), dental calculus represents an impressive long-term reservoir of information regarding the consumption of various alkaloids, whether dietary, medicinal, recreational, or otherwise.


