2 Background


The human mouth, or oral cavity, contains many different types of surfaces on which bacteria can attach and grow. These surfaces are both hard (teeth) and soft (mucosa, tongue, gingiva), and are exposed to the external environment. For this reason, the conditions within the oral cavity can vary considerably, resulting in a unique range of habitats for a wide variety of microbes. In fact, the oral biome contains bacteria from over 700 different species, some of which still haven’t been named, or even cultured. There are so many bacteria in our mouth that it’s actually hard to determine how many there are at any given time, but most estimates are in the billions. Some like stable temperatures and lots of oxygen. Others are better at dealing with fluctuations in temperature and oxygen availability. Some can fend for themselves and take what they need from the environment. Others depend on the presence of other species to break down their food into smaller pieces. Some like acidity. Others like alkalinity. So how can they all seemingly thrive in the same place at the same time? The answer is biofilms.
As an archaeologist, you may be wondering why you need to know all this stuff. Dental calculus is the result of a very complex series of events that involves the physiology of saliva, particular diets, age, genetics, and a bunch of other things. To better understand what we see when we analyse archaeological dental calculus to get at diet, we need to understand all of the processes that went into forming it in the first place. Only then can we begin to fully unlock its potential in reconstructing past diets. In any case, we all have mouths, so on some level I’m sure this knowledge will be relevant.
2.1 Oral biofilms
The concept of biofilms represents a recent paradigm shift in microbiology (Costerton et al., 1987, 1995). Previously, researchers believed that you could isolate the organism of interest and learn about its growth, metabolism, etc. They assumed bacteria would behave the same as a free-floating organism in a lab test tube as it would in a real-world environment (such as the human mouth). More recently researchers have discovered that the behaviour of bacteria differs when they are part of a larger community, compared to when they are grown in isolation. Biofilms consist of large, intricate, multi-species communities of bacteria enclosed in an extracellular matrix of their own creation. The ability to produce this matrix gives the bacteria living within it an adaptive advantage compared to free-floating (planktonic) organisms. It equips them with resistance to both antimicrobials (such as antibiotic medication) and immune responses from the host that would normally be detrimental to their ability to survive (Marsh, 2005; Marsh & Bradshaw, 1997). Resistance to varying conditions is especially important in the oral cavity, which is a site of frequent fluctuations in temperature, pH, and oxygen availability. The viscoelastic nature of the biofilm provides some protection against mechanical destruction and dislodgement caused by, for example, the tongue and dental hygiene practices (Peterson et al., 2015). It also allows them to acquire nutrients from outside the biofilm, as well as generate and distribute nutrients within the biofilm to the various communities of bacteria residing inside (Flemming et al., 2016). Biofilms are quite persistent structures, and very few surfaces exist that can completely prevent bacterial colonisation and biofilm formation (Renner & Weibel, 2011).
2.1.1 Dental plaque
Dental calculus forms from a specific oral biofilm known as dental plaque. After we clean our teeth, our saliva coats the surface of our teeth (enamel) with a layer of proteins known as the dental pellicle (or acquired enamel pellicle). The pellicle is a film that protects our teeth from both mechanical wear and chemical decay, but in doing so, provides a viable surface for microorganisms to attach and initiate biofilm growth (Yao et al., 2003). Biofilm formation goes through several, often arbitrarily defined, stages of growth. They are arbitrary because they are defined by the researchers who study them, but are also necessary as a foundation to explain the development of a biofilm. Rather than thinking about the stages as occurring sequentially, you should think of them as occurring concurrently across different areas of the tooth surface. Biofilm formation is a very dynamic process, and is often over-simplified in visualisations (not unlike Figure 2.1).
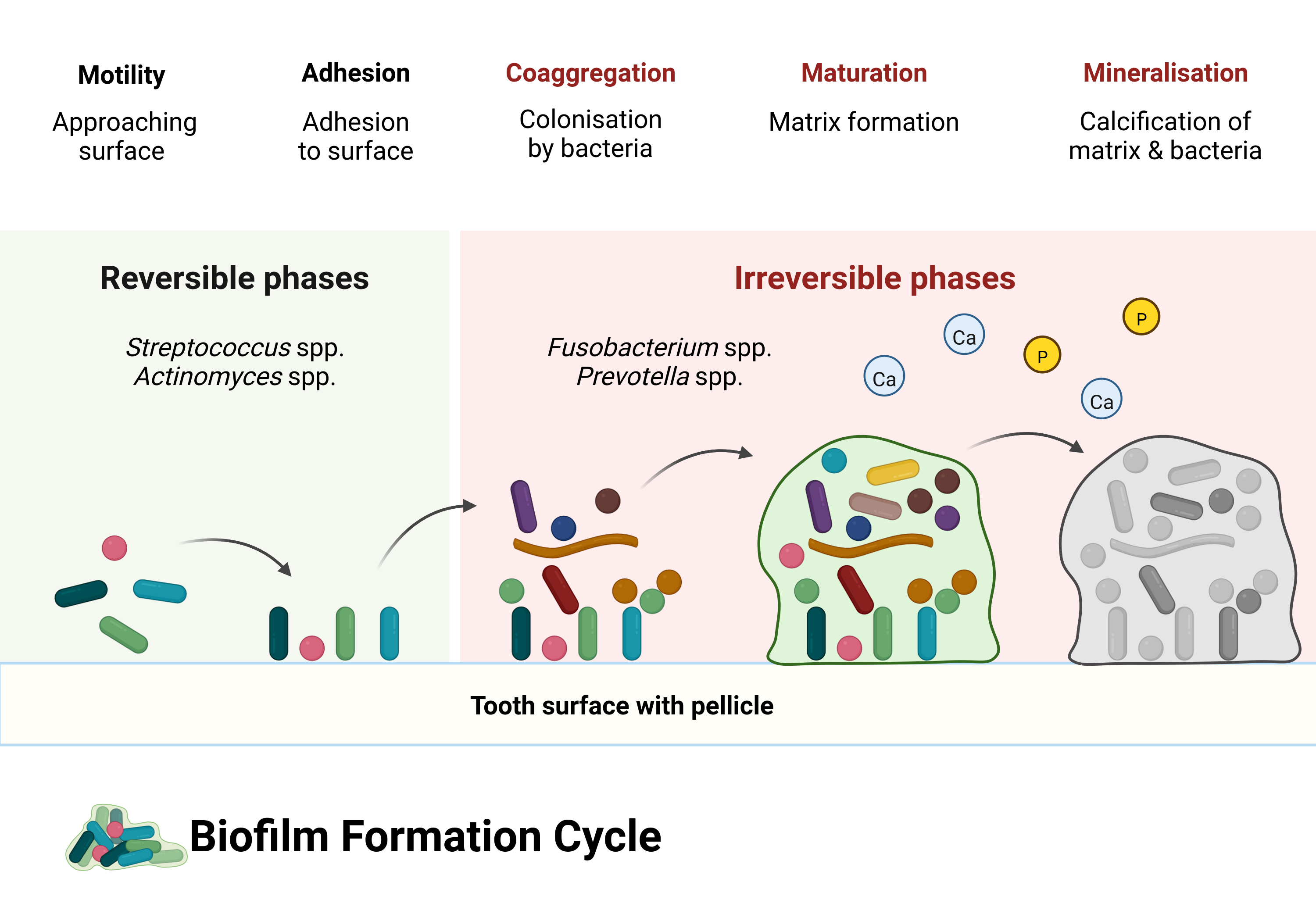
The pellicle contains molecules (known as adhesins) that enable specific bacteria to attach to complementary receptors on the pellicle, in a process called adsorption, not to be confused with absorption. The difference being that it simply attaches to the surface of the tooth rather than being sucked into the tooth. When the pellicle adheres to the tooth, it becomes a surface for bacterial attachment (Yao et al., 2003). The first bacteria to attach are known as early coloniser bacteria (or pioneer colonisers) and include Streptococcus species (spp.), Actinomyces spp., and Haemophilus spp. (Uzel et al., 2011; Zijnge et al., 2010). The initial attachment occurs when the random movement of bacteria and the flow of saliva brings them close enough to the pellicle to attach. Some bacteria have a limited, often random, ability to move if they have long tail-like structures known as flagella, but most are brought to the surface by saliva.
As bacteria approach the pellicle-coated surface of a tooth, there are both attractive and repulsive forces at work. Repulsion because both the bacteria and pellicle proteins have a net negative charge (Song et al., 2015), causing electrostatic repulsive force; and attraction from van der Waals forces. Bacteria may be more or less likely to attach depending on the distance from the bacteria to the surface. If the bacteria come too close to the surface, the initial attraction (primary maximum) will most likely be overcome by repulsion (primary maximum). Bacteria are more likely to attach when they encounter attractive forces at a further distance (secondary minimum), ultimately leading to a game of ‘will-they-won’t-they’ between the bacteria and pellicle. This initial attachment is a weak physicochemical long-distance (10–20 nm; it’s a long distance for bacteria) attraction; therefore, attachment is initially reversible, as bacteria can become detached by salivary flow or shearing action by the tongue (Marsh et al., 2016). This model of bacterial attachment, also known as the DLVO theory, can partially explain the aspects involved in microbial adhesion. Further explanation includes hydrodynamic forces, where hydrophobic components of the pellicle and cell surface interact (Bos, 1999; Vigeant et al., 2002). Overcoming the repulsive forces may be in part facilitated by motility in some organisms. The aforementioned flagellum, for example, may give the necessary ‘push’ to reach a region of net attractive forces (Jin & Yip, 2002). Additionally, the ionic strength of saliva may play a role in reducing electrostatic repulsion with increasing ionic strength (Renner & Weibel, 2011).
Attachment becomes stronger and colonisation becomes more solidified at a shorter distance, as surface molecules on the bacteria interact with complementary receptors on the pellicle, and the interactions between bacteria and pellicle become more direct. Some bacteria have components on their surface that allow them to attach directly to complementary components on the dental pellicle (adhesin-receptor interactions). These attachments are very specific because only certain bacteria have the right molecules on their surface (Jin & Yip, 2002). These receptors are often carbohydrates formed by the host, meaning us. Early colonisers are also able to attach to proteins and enzymes present in saliva, as well as onto the surface of other bacteria already attached to the pellicle (Jin & Yip, 2002; Nikitkova et al., 2013). When bacteria come within a shorter distance of the pellicle they may also attach directly to the surface with other hair-like structures (fimbriae) that are present on the surface of some bacteria. These hair-like structures attach to matching receptors that are present in the pellicle (Nobbs et al., 2009).
While some bacteria specialise in attaching to surfaces, not all of them possess this ability. However, once the specialists have attached, they facilitate the adhesion of other bacteria (secondary colonisers) by allowing them to attach to their surface (coadhesion) rather than directly to the pellicle. For example, Streptococcus gordonii can attach to the pellicle and facilitate coadhesion with Actinomyces naeslundii (Palmer et al., 2003). Not all attachments involve proteins. They can also involve carbohydrates, enzymes, and various appendages on the surface of the bacteria, although these appendages often consist of proteins in their structure, for example the already mentioned pili and fimbriae (Nobbs et al., 2009). This can occur on a large scale, causing the number and types of bacteria on the tooth surface to grow, due to the ability of different species to attach to one another (coaggregation) (Jin & Yip, 2002; Marsh, 2006). Coaggregation and coadhesion are important parts of the growing oral biofilm. Most taxa don’t have the necessary morphology to attach directly to a substrate, however most oral taxa CAN coaggregate with other species through cell-cell interactions, usually involving polysaccharides on the bacterial-cell surfaces (Kolenbrander et al., 2010; Palmer et al., 2017).
As the biofilm formed by early colonisers grows through continued multiplication and coadhesion/coaggregation, the diversity of the biofilm increases. The proportion of early-colonising streptococci gradually decreases while there is an increase of Tannerella forsythia, Actinomyces spp., and Fusobacterium nucleatum (Zijnge et al., 2010). F. nucleatum is a bacterium also known as the ‘bridging species’, as it’s believed to play an important part in linking together early and late coloniser species—including Prevotella spp., S. gordonii, and Porphyromonas gingivalis— which might not otherwise be able to coaggregate (Kolenbrander et al., 2010; Kolenbrander & London, 1993). The increasing diversity of bacteria adhering to a surface results in communities of bacteria with the ability to communicate with each other, distribute nutrients, and alter the local environment for more favourable conditions. This is made possible by the presence of an extracellular matrix, formed by the production of polymers by certain bacterial species (Marsh, 2010). Microenvironmental changes can allow species to survive in otherwise unfavourable environments; for example, the survival of many obligate anaerobes in an environment which is largely aerobic (oxygen continuously enters the oral cavity as we breathe). Bacteria with the ability to consume oxygen and produce carbon dioxide allow bacteria with a lower oxygen tolerance to thrive (Marsh, 2005). In fact, dental plaque predominantly consists of obligate and facultative anaerobes and is especially true for periodontitis-associated biofilms, which tend to be dominated by more species with a lower oxygen tolerance than their non-periodontitis counterparts (Curtis et al., 2020). A pH balance may be maintained by species that are able to consume acidic metabolic products produced by other species, and convert them to weaker acids. Veillonella spp. especially (Marsh, 2005). Metabolic products of some bacteria are used by others as nutrients. By-products of urea metabolism can be used by some organisms, who further break down the by-products, which can be used by yet other organisms (Flemming et al., 2016). Working as a community can increase survivability in the harsh and dynamic environment of the oral cavity, with rapid changes in pH, oxygen, nutrient availability, etc; though, extended fluctuations in environmental conditions can alter the composition of biofilms (Huang et al., 2012, 2017).
Perhaps ironically, an important part of the maturation of a biofilm is the removal of bacteria from the biofilm itself. Removal can occur through both internal and external mechanisms. It’s likely that there is a continuous loss of microbes near/on the surface of the biofilm caused by shear forces from saliva and mechanical removal by the tongue. There can be multiple motivating factors involved in the active detachment by bacteria, including increasingly adverse conditions within the biofilm, such as nutrient depletion or an unfavourable local environment. If sufficiently adverse conditions persist, certain bacteria may make the active decision to ‘peace out’. Dispersion of bacteria from a biofilm requires production of matrix-degrading enzymes, and, as such, not all bacteria can actively disperse from a biofilm (Petrova & Sauer, 2016). The detached bacteria then colonise other parts of the biofilm, making the biofilm a highly dynamic structure undergoing continuous remodelling (Flemming et al., 2016).
So far, the picture of biofilm formation is one of peaceful coexsistence, collaboration, and even neighbourly interspecies actions. A basis for this cooperation is increased overall benefits to the communities (Rendueles & Ghigo, 2015). However, competition between bacteria still exists within the biofilm. The metabolic by-products produced by some bacteria may be toxic for others, allowing the producers to gain a competitive advantage. The aforementioned acid-production by some bacteria can cause unfavourable conditions for species that prefer more neutral pH environments, particularly in the absence of the secondary feeders that would normally neutralise these compounds. A more direct example of bacterial competition is the ability of bacteria to produce substances that are toxic to other bacteria. These are often proteins or peptides termed bacteriocins, and can either inhibit or even kill other bacteria (Daw & Falkiner, 1996; Graham et al., 2017). S. sanguinis and S. gordonii can produce H2O2 that is toxic to S. mutans, a member of their own genus. S. mutans can, in turn, produce mutacin, which inhibits the growth of S. sorbrinus. There is no love lost among these close relatives (Chen et al., 1999). In addition to H2O2, oral streptococci can produce lactate by consuming carbohydrates, giving them a competitive advantage over acid-sensitive species by altering the local environment. Some species are resistant to specific metabolic by-products that others consider toxic, and may even consider them a delicacy (so to speak). Veillonella spp. are an example of organisms that thrive under these conditions, allowing both streptococci and Veillonella spp. to accumulate in the biofilm and create a favourable environment to select species (Edlund et al., 2018). These are simplistic examples, and often competition involves more interactions between multiple species taking on various roles of ‘sensing’, ‘mediating’, and ‘killing’ (Rendueles & Ghigo, 2015). Competition between and within species will ultimately shape the wider biofilm communities.
2.1.2 Dental calculus
The exact mechanism of dental calculus formation is not fully understood, but involves processes of biomineralisation and crystal formation within dental plaque. The main mineral components of calculus are crystals containing various combinations of calcium and phosphate ions. Other salts are also present, but the bulk of the crystals are made up of calcium phosphates. Initial mineralisation of dental plaque is a chemical process in which equilibrium of minerals in saliva and gingival crevicular fluid tips towards saturation with regard to calcium and phosphate, causing an increase of precipitation relative to dissolution. This means that when the concentration of ions increases and tips the balance between dissolution and precipitation, salts will accumulate within and on the surface of the biofilm. An increase in concentration of minerals within the biofilm reaches a critical threshold (supersaturation) and nucleation is triggered within the plaque matrix, initiating crystal growth. This may or may not involve spontaneous (or homogenous) nucleation, as it’s unclear whether mineral concentrations are sufficient to cause spontaneous nucleation, or whether other biochemical processes act as a catalyst (Omelon et al., 2013). That it’s a chemical process can be shown by the ability to produce calculus deposits in germ-free rats (Glas & Krasse, 1962; Theilade et al., 1964). However, it’s unclear how the germ-free calculus compares to conventional calculus, and, to my knowledge there have only been studies on rats. Just because calculus growth can be induced in sterile conditions doesn’t mean bacteria are not an essential part of the process. Bacteria are inevitably part of the scaffolding of dental calculus in humans, since, as I mentioned in the beginning of this chapter, our mouths are full of bacteria, and dental plaque is essentially built by bacteria. Mineralisation does seem to start in the biofilm matrix between microorganisms, but they are eventually also mineralised along with the biofilm matrix (Friskopp, 1983). There are pockets of living bacteria within dental calculus. These pockets and the layer of plaque that covers the surface of dental calculus are likely what cause the correlation between calculus presence and periodontal disease (B. T. K. Tan et al., 2004). While the process can be explained by chemistry, the conditions leading up to and surrounding the process are both chemical and biological in nature, and certainly involve bacteria.
The main source of minerals in the oral cavity is saliva, which enters the mouth through salivary glands. The three main paired glands are the parotid, sublingual, and submandibular glands, located by the cheeks, under the tongue, and under the lower jaw bone, respectively. Saliva contains sodium (Na), potassium (K), calcium (Ca), chlorine (Cl), bicarbonate (buffer), and inorganic phosphate (Pi) (Dawes, 1970; Dodds et al., 2005), and the locations of the glands contribute to the pattern of dental calculus deposits within the mouth, which commonly grow on the buccal portion of maxillary (upper) molars and the lingual portion of mandibular (lower) incisors (Jin & Yip, 2002; White, 1997). Salivary pH also affects saturation of salts, which in turn is influenced by salivary flow rates. Increased flow rate of saliva will increase salivary pH, which reduces dissolution and increases precipitation of calcium and phosphate. This is an important mechanism that protects our teeth against demineralisation of the enamel caused by caries. Protection is provided by the exchange of calcium and phosphate from saliva to enamel (Dahlén et al., 2010). Saliva further acts as a buffer for the oral cavity, reducing the impact of short-term drops in pH caused by metabolic byproducts of acid-producing bacteria (Dodds et al., 2005; Jin & Yip, 2002). Higher rates of salivary flow are also likely to contribute to an increase in calcium and phosphate secretion in addition to pH, all contributing to an environment favouring plaque mineralisation. Metabolic byproducts produced by bacteria can also affect local pH, both pushing towards alkaline conditions as well as acidic. A major cause of acidic pH is metabolism of overabundant dietary sugars and starch, especially the metabolic activity of Streptococcus mutans, known to be one of the main culprits behind dental caries (Bowen et al., 2018; Duarte et al., 2008; Exterkate et al., 2010).
Conversely, alkaline conditions can be generated by metabolism of various products that can either be directly or indirectly linked to diet. One such product is urea. Urea is present in saliva, and its concentration depends on multiple factors. One of these factors is a high-protein diet, which increases levels of urea in serum and saliva (Lieverse, 1999). Hydrolysis of urea produces ammonia and causes a rise in pH. Bacteria possess the ability to produce ammonia from urea, which is further used by ammonia-oxidising organisms and converted to nitrite (Flemming et al., 2016; Sissons et al., 1994; Wong et al., 2002). In a similar way, arginine can be broken down to ammonia and increase in pH. Another pathway to alkalinity is through enzymatic activity. Saliva contains proteases which specialise in breaking down proteins into smaller components such as ammonia, and increased protease activity in saliva may therefore cause an increase in calculus production (Jin & Yip, 2002).
There are also a number of inhibitors and promoters of mineralisation present in the oral cavity, originating both from saliva and bacteria. Substances known to promote plaque mineralisation through hydroxyapatite formation and deposition, calcium-phospholipid-phosphate complexes (CPLX), are present in bacteria. Corynebacterium matruchotii (formerly Bacterionema matruchotii) accumulates calcium within its cell structure, and has therefore received a lot of attention in biomineralisation studies Ennever & Creamer (1967). Biomineralisation is not a feature unique to Corynebacterium matruchotii. Even species associated with caries may induce calcification under the right conditions and after cell death (Moorer et al., 1993; Sidaway, 1978). Inhibitors of biomineralisation include salivary proline-rich polypeptides, small amino acids important for the immune system; and statherin, a protein that controls the precipitation of calcium phosphate in saliva (Jin & Yip, 2002).
It’s likely that multiple biomineralisation events occur under various conditions, resulting in a heterogeneous calculus composition with crystals of various stages of growth (Friskopp, 1983; Friskopp & Hammarström, 1980). The differing susceptibility of bacteria to calcification is also a contributor to the heterogeneous composition. Overall, plaque mineralisation is a complex interaction between conditions in the local environment, availability of minerals, the equilibrium between precipitation and dissolution, balance between nucleation promoters and inhibitors.
2.2 Oral biofilm models
Biofilm models are a way of studying the growth and development of biofilms. By creating models that replicate the conditions and complexity (to some extent) of biofilms in a lab, models allow researchers to conduct various experiments to test the efficacy of treatments on the growth and pathogenicity of biofilms. There are many choices to be made when growing a biofilm, such as the composition of the initial oral microbial community, nutrient content and availability, and the makeup of the atmosphere in which the model is situated. As such, biofilm models can differ widely in their complexity and ability to mimic conditions in a human mouth. A choice of model can be made based on the end-goals of the research, or in some cases the choice is made for you based on (a lack of) available equipment and financial constraints. All models must have a defined biome containing a substratum and nutrients. The substratum is a surface on which the biofilm is intended to form and grow. For oral biofilm models the environment is the oral cavity and the substrata are the teeth, tongue, mucosa, or whatever the model is the biofilm supposed to be mimicking. The simplest models generally involve multiwell plates (e.g., 6-, 24-, and 98-well plates) with a substratum, usually glass cover-slips or hydroxyapatite discs, placed at the bottom of the well. Similar models suspend the substrata from a lid to promote active attachment of bacteria to the substrata (Exterkate et al., 2010). When the substrata are attached to a lid instead of the multiwell plates, it allows samples to be periodically transferred between solutions/media if necessary, adding more flexibility to the experimental setup.
Next, an inoculate is chosen. This can be anything from a single species of bacterium (pure culture), to multiple select species (defined consortium), to all organisms occurring naturally within a system (microcosm) (McBain, 2009). The purpose of the inoculate is to initiate biofilm formation by allowing the bacteria to adsorb to the substrata, ideally in the presence of a conditioning film, such as saliva. For pure cultures and defined consortia, the inoculate may come from saliva or another oral site, such as dental plaque. The bacteria of interest are then isolated using selective media, essentially providing ideal growing conditions to certain types of bacteria, promoting their growth and eliminating others (e.g. Basson & van Wyk, 1996). Alternatively, the bacteria can be acquired directly from companies like the American Type Culture Collection (ATCC). For microcosms, the inoculate is often the saliva itself, or dental plaque, in its (mostly) raw form. The inoculate is added to the wells to initiate biofilm formation on the substrata as described above. As such, the content of the inoculate influences the complexity of the biofilm microbiome as well as the interactions between the communities within the biofilm (Røder et al., 2016). It’s not always possible to use donated saliva as a growth medium for the duration of the experiment, especially if the experiment lasts more than a few days. Media with salivary components can be created as a substitute for long lasting experiments. There are many different recipes for media floating around out there, but most of them are generally a mixture containing mucin, proteins, minerals commonly found in saliva, and a buffer to maintain pH (Exterkate et al., 2010; Pratten et al., 1998; Shellis, 1978; Sissons et al., 1991; Tian et al., 2010).
More complicated models make use of increasingly sophisticated equipment to mimic the oral environment. Another level of model complexity can be added by adjusting the rate at which nutrients are dispersed through the system, and the overall nutrient supply. Nutrient distribution can be continuous, semi-continuous, or batch cultures, with the latter providing a finite amount of nutrients in a closed system. An example of a batch culture model is a biofilm grown on an agar plate, which has a finite amount of resources (Kearns et al., 2005). Once the nutrients in the agar have been depleted, that’s it. At the other end of the spectrum is a system with a pump attached to a reservoir that can continuously supply the biofilm with growth medium, similar to salivary flow. In between the former options is the semi-continuous supply of nutrients. This can, for example, be the multiwell plate model with a lid, where the samples can be periodically transferred to new plates containing fresh growth medium (Exterkate et al., 2010). Other parameters that can be controlled to more closely simulate conditions in the oral cavity are pH and gas phase, as can be done with the multistation artificial mouth. This system gives researchers control over a large number of parameters using multiple chambers with complete control over the flow of treatment and/or nutrient conditions—environmental conditions such as pH, temperature, and gas phase—and access to real-time measurements (Sissons, 1997).
The duration of an experiment depends on the scope of the study. If the purpose is to learn more about initial biofilm formation and prevention, it may only be necessary to grow the biofilms for a few hours to 48 hours (Dibdin, 1981; Exterkate et al., 2010). If, instead, the goal is to learn more about biofilm maturation and calcification, the experiments can run for days or even weeks (Filoche et al., 2007; Sissons et al., 1991; Wong et al., 2002).
Models developed for studying oral biofilms include, in increasing complexity, the ACTA active attachment model (Exterkate et al., 2010), Calgary biofilm device (Ceri et al., 1999), modified Robbins device (Honraet & Nelis, 2006), constant depth film-fermenter (Peters & Wimpenny, 1988), and the multistation artificial mouth (Sissons et al., 1991) representing the upper echelon of complexity. Summaries of biofilm models, including benefits and limitations of the various types, can be found in reviews by McBain -McBain (2009), Tan and colleagues -C. H. Tan et al. (2017), and Røder and colleagues -Røder et al. (2016).
It might be tempting to think that the goal should always be to mimic the oral environment as closely as possible. However, there are benefits to more simplistic models, as well as limitations to the more sophisticated models. Benefits of pure cultures and defined consortia are reproducibility between experiments and more control over physiological and factors and making it easier to take various measurements. Microcosms have the benefit of more closely mimicking the complexity of the organisms’ natural environment (McBain, 2009). However, even microcosms can be limited in their ability to recreate the complexity and diversity of the oral microbiome (Tian et al., 2010). Alternatives to in vitro models are in situ models which usually involve growing plaque on a removable surface inside the mouth of a willing participant. These models add a level of realism, as they are grown inside an actual oral cavity, and can reflect biogeographical differences in biofilm composition caused by differing conditions across the oral cavity. They also come with additional difficulties and reduced control over experimental parameters (Marsh, 1995; Zero, 1995).
Reiterating a point made in the Introduction, and Discussion, and probably somewhere in the articles as well, the benefit of using an oral biofilm model over naturally occurring dental calculus in the mouth of a research participant, is the control that it provides to tweak every aspect of the system, from the quantity and quality of nutrients available, to the amount of enzymes and bacterial species present. Plus, the added ethical benefit of not needing to ask someone to give up their oral hygiene regime for a few weeks. The following chapters, Chapter 3 and Chapter 4, provide a small glimpse of what a model looks like, and how it might be used to inform archaeological research.