1 Introduction


Dental calculus is becoming a popular substance in research on the behaviour and biology of people in the past. You may also know it as tartar or mineralised plaque. In other languages the word is often related to “tooth stones”. In fact, calculus is itself latin for ‘pebble’. This was originally used as a term for mathematical calculations using counting stones, and only later used to describe various calcifications in the human body (https://www.etymonline.com/word/calculus). This can be the cause of some confusion, as calculus is also a branch of mathematics. If you see the term ‘calculus’ in this dissertation, you can safely assume that I’m referring to stuff that grows on your teeth and for which you receive lectures from your dentist, and not the topic you dreaded in high school.
I will briefly describe the formation of dental calculus here, but for a more thorough review of the entire process I refer you to Chapter 2. Dental calculus is formed from dental plaque, a substance that grows on your teeth and consists mainly of bacteria and a surrounding structure called the extracellular matrix. When the local environment within and around the plaque reaches a favourable alkaline pH, both the extracellular matrix and bacteria within will calcify (Jin & Yip, 2002; D. J. White, 1997). The alkaline pH causes minerals (especially calcium and phosphate) from saliva to enter the plaque, causing the extracellular matrix and eventually also the bacteria to harden, resulting in a concrete-like deposit on the surface of the teeth. The process repeats itself when new bacteria colonise the surface of the newly formed dental calculus, creating a layered structure, though somewhat disorganised (Akcalı & Lang, 2018; Jepsen et al., 2011). Dental plaque can accumulate more easily on teeth (and dental calculus) because they are a hard, non-shedding surface. Most of the surfaces in our mouth are covered by a layer of cells called the oral epithelium. These cells are continuously renewed as new cells are formed and dead cells fall off (Squier & Finkelstein, 1998). This constant turnover means that it is difficult for bacteria to build the communities they require for producing biofilms. Enamel, the white substance that covers the crown of your teeth, behaves differently. It stops growing when the tooth has fully formed. After that, there is no renewal. This allows bacteria to continue to grow and develop communities if there is no intervention from you (or your dentist). Dental plaque can trap a variety of different microparticles, including bacteria, human proteins, and small debris from the food we eat (De La Fuente et al., 2013; Hendy et al., 2018; Henry & Piperno, 2008). When the plaque mineralises, it can preserve these microparticles over long periods of time, even after the person whose teeth provided a home for the calculus has died. Also, the main crystal structures in calculus strongly bind DNA, making calculus a fantastic source of ancient DNA (aDNA) from the mouth (Warinner et al., 2015). Another advantage of dental calculus is that it represents a more recent and direct source of diet than teeth or other bones. While bones and teeth can take years to remodel and incorporate a dietary signal, calculus forms on a much smaller timescale and is in direct contact with the dietary material. Calculus can form within weeks at any point during an individual’s life and may, therefore, indicate a recent and direct consumption of food, while bone can take years to show a (indirect) dietary signal, following food molecules entering the bloodstream, and finally entering the bone from there. Further, enamel stops forming after the crown of the last tooth has developed—third molars, or ’wisdom teeth—at around 16 years of age, and the turnover of dentin is very limited (Hillson, 1996). These properties are probably why archaeologists have become increasingly interested in dental calculus.
1.1 Dental calculus in archaeology
The main archaeological interest in dental calculus is to explore research questions involving diet and the evolution of the oral biome and oral health. To this end, it can contribute a surprising amount for such a small, seemingly insignificant material. This relates to its ability to retain and preserve a wide variety of different materials, from the food we eat to the bacteria that make their home in our mouths (Adler et al., 2013; Fellows Yates et al., 2021; Henry & Piperno, 2008; Warinner, Rodrigues, et al., 2014; Warinner, Hendy, et al., 2014). The goal of current studies targeting archaeological dental calculus have not changed much since the early uses of dental calculus in archaeological research, but the methods certainly have, allowing us to unearth information that was previously not considered possible. By my count, archaeological dental calculus has now been subject to various forms of microscopy (Charlier et al., 2010; Middleton & Rovner, 1994; Robert C. Power et al., 2022); extractions of biomolecules including DNA, proteins, and metabolites (Adler et al., 2013; Warinner, Hendy, et al., 2014); and stable isotope analyses.

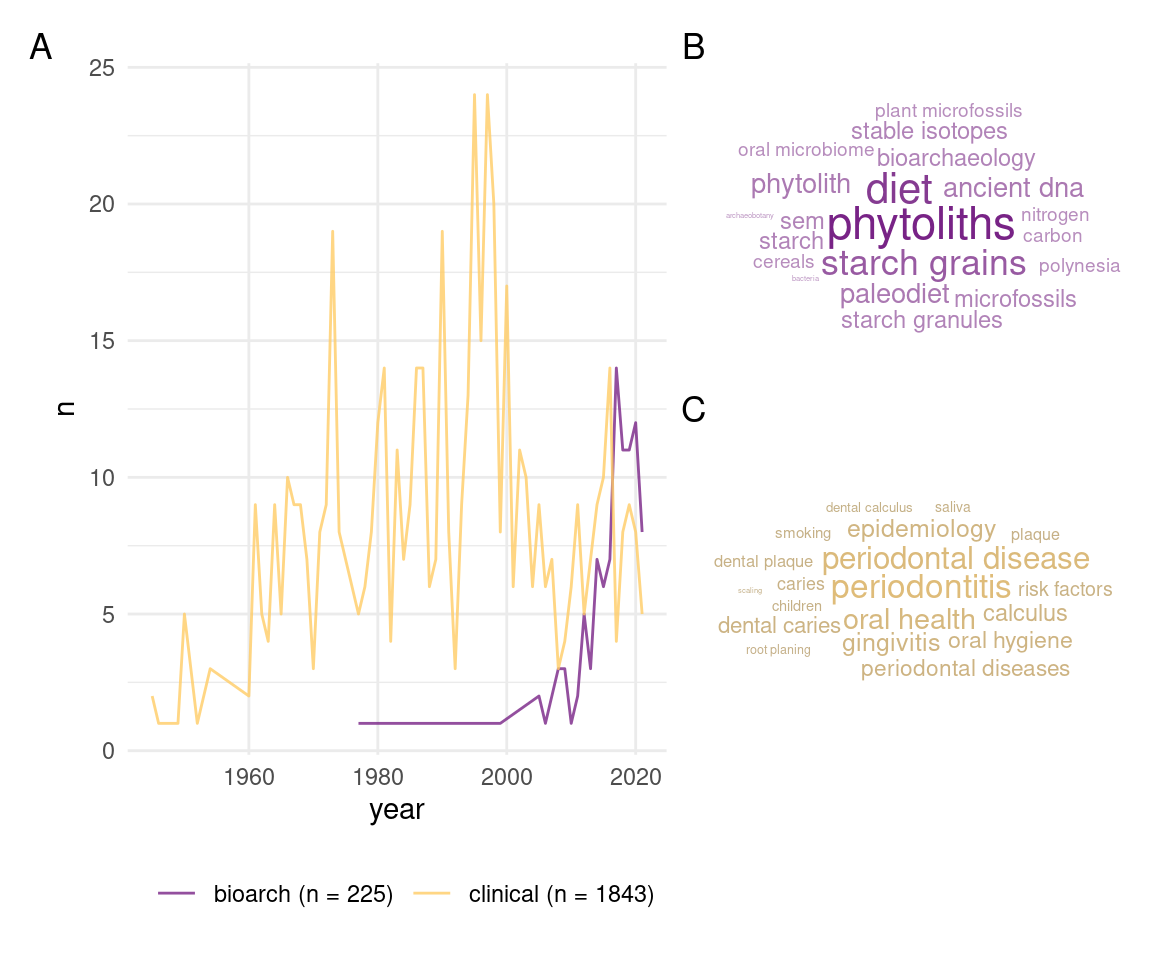
Perhaps the most common use of dental calculus is to recreate the diet of past people and populations (Figure 1.1B). One of the ways to do this is by dissolving the calculus in a weak acid or decalcifant, or mechanically breaking it up. This process releases any fragments of plants that were trapped within the calculus and can be identified, for example with a microscope. The tricky part is not destroying the plant fragments when releasing them from the calculus. As far as I can tell, the first attempt at this was the extraction of phytoliths (silicified plant remains) from the teeth of cows, sheep, and horses (Armitage, 1975). This was a somewhat isolated use-case, and the method didn’t really catch on until the 1990s (Ciochon et al., 1990; Middleton 1990, in Middleton & Rovner, 1994). The first extractions from human teeth followed shortly (Fox et al., 1996), and there are now studies using plant microremains (especially starch granules and phytoliths) from dental calculus to infer diet in past peoples from across the world, including Pacific Islands (Dudgeon & Tromp, 2014), China (Chen et al., 2021), Europe (Fiorin et al., 2021), and more (Buckley et al., 2014; Henry & Piperno, 2008; Mickleburgh & Pagán-Jiménez, 2012). The durable nature of dental calculus also means that microremains within it can survive for millennia, allowing us to look at the diets of early humans and other hominins (Buckley et al., 2014; Chen et al., 2021; Hardy et al., 2009; Hardy et al., 2012; Henry et al., 2012, 2014; Henry & Piperno, 2008; Piperno & Dillehay, 2008).
That bacteria can become trapped within calculus has been known to archaeologists for a while (Brothwell, 1981, ; Vandermeersch et al., 1994), but it wasn’t used in archaeological research until DNA extraction started to become more accessible (De La Fuente et al., 2013). Dental calculus then became part of the third scientific revolution in archaeology. The early studies focused on oral health in the past (Adler et al., 2013; De La Fuente et al., 2013; Warinner, Rodrigues, et al., 2014). Bacteria have shorter lifespans than humans which makes them useful when studying the evolution of bacteria in the human mouth (De La Fuente et al., 2013; Fellows Yates et al., 2021). Diet has also been a focus of paleogenetic research. This has mainly been addressed by considering how long-term changes in the patterns of bacteria within the mouths of our ancestors have changed that could be related to changes in diet. Just like we adapt to deal with various diseases, climates, etc., we also adapt to changes in our diet (Adler et al., 2013; Fellows Yates et al., 2021). Directly identifying genetic markers of plants and animals within dental calculus is difficult, but not impossible (see Warinner, Hendy, et al. (2014)). Most of the DNA within dental calculus will be oral bacteria, and this will overwhelm the small signal from plant DNA, which makes species identifications problematic (Fagernäs et al., 2022). A newer field of biomolecular archaeology, paleoproteomics, may be able to address this issue by targeting plant proteins, along with a range of other dietary protein sources. Hendy and coauthors were able to identify a number of these in dental calculus, as well as proteins from cereals, and milk proteins from different sources (Hendy et al., 2018). Dental calculus has also become a target for extracting other biomolecules that may be related to diet, such as alkaloids, fatty acids, and carbohydrates (Gismondi et al., 2020; Velsko et al., 2017). The methods used for this have also proven to be useful in detecting compounds that are related to other activities and ceremonies, such as nicotine (Eerkens et al., 2018), and may provide some evidence of medicinal practices (Gismondi et al., 2020).
To a lesser extent, the presence and amount of dental calculus on teeth has been used as an indicator of dental health (Drewett, 1975; Lieverse et al., 2007; Sagne & Olsson, 1977; Zhang, 1982). Pilloud & Fancher (2019) explored the terms associated with a number publications on dental or oral health, dental calculus came up as one of them; albeit not the most common, which was (unsurprisingly) dental caries (Figure 1.2).
To a lesser, lesser extent, it has also provided some interesting insights on non-dietary activities, such as occupations and smoking habits. In a rare find, blue particles were detected in the dental calculus of a Medieval German woman. These blue particles originated from lapis lazuli, an exotic stone often ground into pigments and used to illuminate manuscripts (Radini et al., 2019). Nicotine was detected in dental calculus of pre-colonisation individuals from California using Ultra-Performance Liquid Chromatography Mass Spectrometry (UPLC-MS), showing direct consumption of tobacco and providing more detailed insights on the demographics of consumption in a way that no other human-adjacent archaeological materials can.

It wasn’t always appreciated for the wealth of information hidden within its hardened shell. Until roughly 20 years ago, archaeologists who encountered calculus had limited use for this material. Some researchers quantified it using a simple four-stage scoring method that was developed for recording deposits on archaeological dental calculus (Brothwell, 1981), similar to a common clinical scoring system (J. G. Greene & Vermillion, 1964). The four-stage system is probably still the most widely used among archaeologists. More detailed methods are also available (Dobney & Brothwell, 1987; T. R. Greene et al., 2005), but the original method is generally preferred for its simplicity. Unfortunately, knowing the size of a calculus deposit is not as valuable as being able to analyse the deposit itself, and the deposits were often removed because they obscured tooth and root morphology (Scott, 2015). This had made a lot of people very angry and been widely regarded as a bad move (Adams, 2002, p. 1). Hindsight being what it is, it’s hard to blame anyone. A lot of dental research mainly focuses on the prevention and removal of dental calculus.
The wide range of applications for dental calculus that we know about today, and the fact that it’s pretty much ubiquitous in the past thanks to poor oral hygiene, makes it a really exciting target for future (and current) paleodietary research. That being said, the study of dental calculus doesn’t seem to fit into any predefined areas of study within (and beyond) archaeology. Most researchers seem to see it as a means to the information contained within, rather than being worth studying in its own right. This can be problematic. Other than what we can see with our current methods, what do we really know about dental calculus and how its growth and structure affect the reliability of these methods and potentially distort our interpretations of the past?
1.2 What is dental calculus?
To answer these questions, we must first answer a single, surprisingly difficult question: What is dental calculus? I’m not referring to its formation or composition, which I briefly described above. How do we categorise it? Is it a dental disease? An oral health condition? A byproduct of oral conditions? We start by exploring various definitions of oral health. Definitions in an introduction are a little cliché and tedious, but often necessary. Since oral health is a complex topic, definitions of oral health are often purposefully (and confusingly) broad, and they extend beyond physical well-being and into the realms of emotional and social comfort. The World Dental Federation (FDI) defines oral health as the ability to perform mouth- and face-related functions with confidence and without pain (including smiling, speaking, eating, etc.) (“FDI’s Definition of Oral Health | FDI,” n.d.) (https://www.fdiworlddental.org/fdis-definition-oral-health). Both the World Health Organisation (WHO) and FDI take a similar approach to defining oral conditions, giving a list of conditions that cause discomfort, pain, disfigurement, or death. The list includes the dental conditions tooth decay (caries), gum disease (periodontal disease), and dental trauma, but not dental calculus (“Oral Health,” n.d.) (https://www.who.int/news-room/fact-sheets/detail/oral-health). While these are not likely to cause death, they are often the source of physical and emotional discomfort, and may cause further health complications if they are not dealt with in a timely fashion.
Dental calculus and dental plaque are not considered oral conditions according to WHO. In fact, dental plaque is part of the normal functioning of our oral biome (Marsh, 2006). When plaque reaches a certain level of acidity over a prolonged period of time, the normal functioning of the bacteria within the plaque may shift towards a disease-causing function. The biofilm will cause the surface of the enamel to demineralise, eventually resulting in a cavity (or caries). Dental caries are unequivocally considered a dental disease. If, instead, the biofilm calcifies, dental calculus is the result. Its status in oral health is questionable.
Dental calculus is not known to be painful, nor does it affect the ability to perform the functions listed above. However, with continued accumulation, it may affect the confidence of the person performing these tasks (Collins & Freeman, 2007), and in extreme cases it can affect function (Balaji et al., 2019). Most of the virulence and disease-causing potential is lost when the bacteria within dental plaque calcify (Akcalı & Lang, 2018). It has been shown to contain pockets of living bacteria that can be detrimental to oral and dental health (Tan, Gillam, et al., 2004; Tan, Mordan, et al., 2004). The rough, porous surface of dental calculus is also a great place for bacteria to attach more easily and develop a new layer of plaque on the surface of the calculus. This is likely why there is often a correlation (NOT causation) between dental calculus and periodontitis, especially subgingival calculus (Jepsen et al., 2011; D. J. White, 1997). Since it seems to fulfill some of the criteria of an oral condition, it should be considered as such, at least under the definitions provided by WHO and FDI. Whether or not dental calculus can be considered an oral disease is more questionable. While it does grow on the surface of teeth, it doesn’t seem to affect the underlying enamel. And while there is a relationship with periodontal disease (which has been defined as a dental disease), the nature of this relationship is still under debate, with calculus likely being a secondary contributor (Jepsen et al., 2011). As such, we can probably limit the definition to an oral condition and not necessarily a dental disease (Pilloud & Fancher, 2019). In fact, dental calculus is quite hard, so a layer of dental calculus on a tooth can actually protect it from wearing down (although there are better options).
1.3 The study of dental calculus
It seems that the researchers who are studying dental calculus approach it from a wide range of different fields and backgrounds, including genetics, proteomics, botany, and (bio)archaeology. The paleogeneticists mine it for the wealth of information it contains on oral health and disease in the past (Fellows Yates et al., 2021; Warinner, Rodrigues, et al., 2014). Paleodiet researchers extract microremains and residues from food (Henry & Piperno, 2008; Mickleburgh & Pagán-Jiménez, 2012) to infer dietary practices. Bioarchaeologists use its presence and amount to broadly infer diet, and dental and overall health in a given population (Belcastro et al., 2007; Lieverse et al., 2007; Novak, 2015; Šlaus et al., 2011; Yaussy & DeWitte, 2019). This leaves research output from studies of calculus scattered across multiple venues, with no clear gathering point. I think it’s fair to say that dental calculus should be included in discussions of pathological oral conditions, even if its role is secondary. But who is currently studying dental calculus as a substance in its own right? And why do we need to learn more about it if we’re just interested in what’s inside? Related discussions have started to take place in recent years (Bucchi et al., 2019; Radini & Nikita, 2022; Wright et al., 2021).
The lack of a specific field of study for dental calculus to belong may be related to how it’s taught to students (and if it’s taught at all). Textbooks from the more established fields in bioarchaeology are probably a good indicator of the teaching curricula, which also impacts research focus. The most popular osteoarchaeology textbooks only briefly mention dental calculus as more of a footnote than anything else. A couple of lines describing what it is (usually ‘mineralised plaque’) and that it can contain food debris and bacteria T. D. White et al. (2011). They’re not wrong. Diseases that manifest themselves in the skeleton as lesions on the bones have a very clear home in paleopathology. No one questions whether or not the degeneration of vertebrae from tuberculosis should be included in the paleopathology textbooks (at least not as far as I’m aware).
These textbooks often include chapters on dental disease, where more detailed descriptions of dental calculus are usually found (e.g. Roberts & Manchester, 2007; Waldron, 2020). Dental caries, calculus’ more famous sibling, will often get a few pages. In some cases, dental calculus may even be hidden within a section on periodontal disease or plaque (Aufderheide et al., 1998; e.g. Ortner, 2003). The focus of these (sub)sections is varied, with some simply describing what it is, and others giving brief discussion on the relationship between calculus and periodontal disease. A more detailed section was dedicated to dental calculus in Ortner’s Identification of Pathological Conditions in Human Skeletal Remains, with a detailed description of formation, structure, and application in (biomolecular) archaeology (Kinaston et al., 2019). The description extends well beyond any (paleo)pathological significance of dental calculus. Can we fault the authors/editors for not giving it more attention? After all, it’s not a dental disease, and its relationship with other dental diseases is unclear. What is clear, is that it has implications for oral health, and, for that very reason, could be addressed more extensively in paleopathology; certainly in the textbooks that include dental disease.
On the surface, dental anthropology seems like a more suitable home for the study of dental calculus. However, it’s not included in A Companion to Dental Anthropology, an otherwise great resource on studying archaeological teeth. The editors briefly acknowledge the valuable information gained from calculus and that it holds a lot of potential; but that’s it (Scott, 2015). Other notable absences include textbooks such as Technique and Application in Dental Anthropology and New Direction in Dental Anthropology (Townsend et al., 2012), both of which dedicate considerable attention to dental caries. Hillson’s Dental Anthropology, a book that I consider to be the ‘bible’ for dental anthropology, has a section on dental calculus in the Dental Disease chapter. It covers a basic description, the composition, microscopic structure, methods used for recording archaeological calculus, and the distribution in the dentition (i.e. which teeth are more prone to calculus buildup) (Hillson, 1996). Considering these are entire books devoted to the dentition, it seems odd that there is often only a few paragraphs (if that) on dental calculus. Granted, the only function teeth serve in the growth of dental calculus is as a suitable surface on which to attach; though the role of substratum is an important role, as dental calculus is seemingly unable to form on other surfaces in the oral cavity.
Since the use of dental calculus in biomolecular archaeology is relatively new, there are fewer available textbooks, and it rarely has a dedicated course. The most common place to find descriptions of dental calculus is, therefore, journal articles. There will be a short paragraph on dental calculus formation (and sometimes composition) in the introduction section. These are quite variable and are often limited by the word count of the journal. Despite this, the descriptions will often be as long, if not longer, than the sections in textbooks devoted to dental calculus (Velsko et al., 2019). The focus of these paragraphs are generally the same. They describe the formation and mineral composition of dental calculus, and provide some examples of how dental calculus has been used in related studies (not unlike the beginning of this chapter). The contribution of dental calculus to archaeology has been significant, so it is likely to receive more and more attention going forward. In fact, an entire chapter was recently devoted to dental calculus in the second edition of Handbook of Archaeological Sciences (Fagernäs & Warinner, 2023). Take that, dental caries!
1.4 The challenges of studying dental calculus
What we know about dental calculus and the influence of diet was reviewed in an article aimed at (bio)archaeologists. The overall conclusion reached in the article: it’s still pretty unclear (Lieverse, 1999). Now, 20-some years later, there has been limited progress on this point. High-protein diets are linked to an increase of urea, which is linked to an increase in oral pH, which is linked to mineral deposition (Dibdin & Dawes, 1998; Wong et al., 2002). BUT, protein may also inhibit crystalisation (S. Hidaka & Oishi, 2007). Starch consumption has been linked to increased rates of caries in early farming populations (Storey, 1986). This is consistent with in vitro testing, at least for starches high in amylose content. So a high-starch diet causes caries, not calculus, right? Well, starches with a high amylopectin content are linked to increased calcification (S. Hidaka & Oishi, 2007). It likely depends on what is consumed along with the starch (Saburo Hidaka et al., 2008). There is also some (in vitro) evidence to suggest that silica may promote dental calculus formation by promoting mineral precipitation, i.e. the transfer of minerals from saliva to the biofilm (Damen & Ten Cate, 1989). Overall, this is an understudied area in both clinical and archaeological contexts.
Another aspect of diet and dental calculus where we are still looking for answers, is the process that causes fragments of food and other environmental materials to become entrapped in the dental calculus. We know that it happens. Decades of research has shown dental calculus to be a seemingly unlimited resource for dietary substances. We don’t know exactly how this happens, and herein lies the potential for bias. Efforts have been made to understand how much of the consumed food makes it into the calculus. These include studies on modern humans (Leonard et al., 2015) and non-human primates (R. C. Power et al., 2015; Robert C. Power et al., 2021), where food intake is meticulously documented, and calculus subsequently analysed. These studies have common findings; the amount of the diet that becomes trapped in the dental calculus of any one person has no clear relationship to the amount of food that was consumed. The most likely reason is that the formation of dental calculus differs between people (R. C. Power et al., 2015). So, it’s not a great way to study the diet of a single person, but generally suitable to study patterns in the diet of a population. The more people you study, the more likely you are to gain a complete picture of the diet in a population. The fact that we can still see (in some cases, literally) remains that were consumed thousands of years ago is pretty cool. We just need a better understanding of why the record of diet from dental calculus differs from the actual intake of food. This will allow us to make more robust interpretations about past dietary practices. Something that may influence the dietary record that we get from calculus is the method we use to extract the dietary remains from calculus. Our understanding of dental calculus extraction methods is improving, with studies looking at the effect of various acids used to dissolve calculus (commonly EDTA or HCl) (Bucchi et al., 2019; Palmer et al., 2021; Soto et al., 2019; Tromp et al., 2017); as is our understanding of how the choice of tooth may affect our results (Fagernäs et al., 2021), and that not all we see is related to deliberate consumption (Delaney et al., 2023).
These studies provide valuable insights into potential biases of our sampling methods and the representation of diet within dental calculus, with a minor caveat. Most of these studies have been conducted on living primates or archaeological remains. An issue with using living (or once living) organisms is the inability to control factors related to the variability between subjects. Basically, studying humans is messy and complicated because we’re all unique. It’s a lovely sentiment but it can make for some messy science. Not bad science (not at all!). Just messy. A method of study that offers more control is the growth of plaque and calculus in a lab. This allows us to control many of the things that are difficult to control in humans, such as the bacteria that colonise our mouth, where each person has a pretty unique makeup of bacteria. We also have a very unique genome (with the exception of identical twins) that plays a role in how quickly we form calculus in our mouth (if at all). Certain enzymes start digesting our food as soon as it enters our mouth, and the activity of these enzymes fluctuates throughout the day, causing a lot of variability both within and between individuals. Finally, the number of microremains that enter our mouth over days, weeks, and months, can be very different between people, even with the same diet. All these things can muddy the results of research on living subjects, where a lab-grown approach can help tease out confounding factors. I don’t believe research conducted on lab-grown biofilms can in any way replace studies with modern or archaeological individuals, nor should they. But it can complement these studies by zooming in on certain aspects that are too difficult to isolate in (once-)living people.
Often we can draw from clinical studies as there are common goals, e.g. discovering the aetiology and/or presentation of a disease. However, the motivation driving the studies in archaeology and dental research are inherently different; although, there is certainly overlap in some areas (Figure 1.1B and C). There is more interest in preventing dental calculus from forming in the first place, so most studies focus on short-duration models to explore anti-microbial treatments and inhibition of biofilm formation and plaque buildup (Exterkate et al., 2010). As shown in a previous study, calculus and plaque have distinct microbial profiles (Velsko et al., 2019), so the applicability of short-term models to explore archaeological questions on dental calculus are limited, since plaque is rarely (if ever) preserved. Archaeologists are more interested in questions related to how diet influences the growth of biofilms, and how fragments become embedded inside, and what we can say about diet. Further, the interest in dental calculus as a field of clinical research has been declining since the 2000s, which, as far as I’m aware, is when the last studies growing dental calculus in a lab were conducted. We can see this by the number of clinical articles with the term dental calculus in the title (Figure 1.1A). And they certainly aren’t interested in how food debris becomes trapped inside our calculus. Dental calculus has also become less of a problem with the use of modern dental hygiene practices and regular visits to the dentist (Velsko et al., 2019).
To summarise: Bioarchaeologists are interested in how dental calculus relates to dental and general health; paleodietary researchers are interested in the food remains that are trapped inside; paleogeneticists are interested in accessing the oral bacteria that have been fossilised within; clinial dentistry views it as a nuisance to be removed and, ideally, prevented from forming in the first place. This lack of systematic research specifically devoted to dental calculus as a substance, rather than a means to an end, leaves a lot of questions regarding the expected behaviour of dental calculus and how information from the past becomes trapped inside. To summarise the summary: we need to ask more basic questions about dental calculus.
1.5 Aims
This dissertation is a contribution to a dental-calculus-centric body of knowledge, and addresses a gap in the fundamental research on dental calculus to further our understanding of how we can use dental calculus to reconstruct the diets of people in the past. The main aim is the development, validation, and application of a calcifying oral biofilm model to improve interpretations on archaeological dental calculus. By developing a model system we can isolate the effects of confounding factors in dental calculus and diet, and explore new uses for dental calculus in paleodietary reconstructions through fundamental experimentation. I also aim to assess the potential and limitations of dental calculus to explore dietary activities of past populations.
Every decision we make, from sampling to statistical analysis, leads us down a unique path towards a different interpretation from the other possible paths in the multiverse of analyses. It’s important we fully understand the path we take, to ensure that it is the right path given the limitations, and one that maximises the validity and detail of our interpretations.
With these aims, I hope to address the following research questions:
How can we improve the resolution of our interpretations using dental calculus on individuals and populations? We are stuck in the identification of compounds, and unable to speak to the quantity, since we know that it’s not very representative of a single individual.
Can we trust the system? (i.e., using dental calculus to reconstruct diet) Since we don’t know the mechanism of incorporation, there are likely hidden biases and limitations of our methods as a result. We don’t know the starting point, i.e., exactly what and how much was originally trapped inside, so we have difficulty validating our methods.
How can a model improve our understanding of dietary reconstructions using dental calculus? How can it address current challenges in paleodietary reconstructions, and can it help us produce a better understanding of how dietary intake relates to the record of diet we extract from archaeological dental calculus?
1.6 Thesis outline and structure
If you have made it to this point, you have probably read most of Chapter 1, in which I provide some context to the study of dental calculus in archaeology and identify some areas that could benefit from further investigation. Chapter 2 provides some background information on oral biofilms and oral biofilm models in more detail than I can do in the research articles included in Chapters 3 and 4. So if you’re already well-versed in oral microbiology, feel free to skip to Chapter 3. If not, I recommend picking up a textbook written by actual experts in the field of oral microbiology. If, for some reason, you can’t access one of these, feel free to read Chapter 2. I suppose there are worse options than something written by a PhD student in archaeology. The chapter reflects the current knowledge of biofilms and the oral microbiome (as best I could summarise) at the time of writing, and no warranty is given for the inevitable new developments that will change what we now believe to be true.
To address the aims of the dissertation outlined above, I developed a protocol to grow dental calculus in a lab on plastic tubes instead of looking at the real stuff you normally find inside your mouth. The reason for using lab-grown biofilms instead of humans is that the in vitro lab model offers more control over all the factors that go into the growth of dental calculus, at least in theory. The real world is messy, and sometimes you need to remove things from the real world to break it down and really get into the nitty gritty of how it works. There are many different kinds of biofilm models, including single species of bacteria, select species determined by the researchers (defined consortium), and multiple species from some natural source (the human mouth, for example). I will cover the different types of models in more detail in Chapter 2. Since there are many biofilm models to choose from, developing a new protocol may seem counter-productive; however, few are developed for long-term growth and even fewer with the purpose of mineralising the biofilm to form dental calculus. One of the exceptions involves a highly complex setup that is unlikely to be supported by budgets and facilities available to most archaeological laboratories (Sissons et al., 1991).
After developing a working protocol, the next step was to determine if the stuff I grew in the lab is actually dental calculus. Or at least something close enough that we can use it to explore our research questions. To do this, we (myself and coauthors) determined the mineral and bacterial composition of our model using Fourier Transform Infrared (FTIR) spectroscopy and metagenomic classification Chapter 3. We then compared the results of these analyses to naturally grown dental calculus, both modern and archaeological.
Being confident that our model looks and behaves like human dental calculus, we then set out to test some very basic behaviours of starch grains within dental calculus. Chapter 4 is a research article where we ‘fed’ the biofilm with a known quantity of starch granules during the growth period to see if the input quantity/ratio matched the extracted quantity (or output). Those who are familiar with dental calculus research will not be surprised that it did not. The more interesting outcome of the study is the more detailed explanation of how the input and output starch quantities were mismatched.
Chapter 5 is a separate article, in the sense that it doesn’t involve the biofilm model in any way. Rather, it addresses the theme of the overall utility of dental calculus in archaeological research. We look at possible medicinal compounds in the dental calculus of a Post-medieval Dutch population. We employed Ultra High Performance Liquid Chromatography coupled with tandem Mass Spectrometry (UHPLC-MS/MS) to identify various compounds in dental calculus, including alkaloids and other compounds. It shows the potential of dental calculus to inform about past practices, but also highlights some of the limitations we are currently experiencing in the field. Chapter 6 is a discussion on the limitations and future potential of dental calculus in the field of archaeology, and what biofilm models can contribute to our understanding of past diet.